A&H Focal Inc. Issues Nationwide Recall of 21 Products Marketed as Dietary Supplements & One Cosmetic Product Due to Undeclared Drug Ingredients
This is a reprint of an FDA Alert.
March 7, 2017
Contact
Consumers
Henry Choo:
(646)327-8522
A&H Focal Inc. is voluntarily recalling all lots of the following products because many of these products have been historically tested by the FDA and found to contain PDE-5 Inhibitors (i.e. sildenafil, tadalafil, vardenafil, etc.) which is the active ingredient in an FDA-approved drug for erectile dysfunction (ED) making these tainted dietary supplements unapproved drugs.
These undeclared active ingredients poses a threat to consumers because the PDE-5 Inhibitors may interact with nitrates found in some prescription drugs such as nitroglycerin and may lower blood pressure to dangerous levels. Consumers with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates.
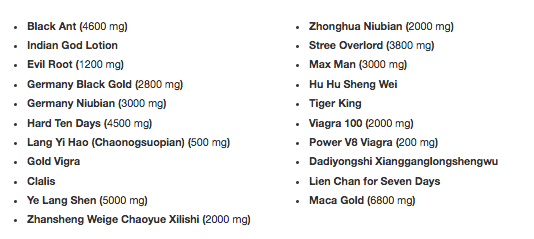
These products were marketed as dietary supplements for male sexual enhancement. All lots of the listed products sold by A&H Focal Inc. since January 2014 to present are included in this recall. The products were mainly sold through Asian Markets located in NJ and NY.
Consumers who have any of the above mentioned products should immediately stop use of the product and properly discard. If you have further distributed this product please notify those individuals of this recall.
Consumers with questions regarding this recall can contact Mr. Henry Choo by calling 646-327-8522, Monday through Saturday, 9am-6pm, EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using these drug products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report
- online: www.fda.gov/medwatch/report.htm
- Regular Mail or FAX: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request reporting form, then complete and return to the address on the pre-addresses form, or submit by fax to 1-800-FDA-0178.
This recall and market action are being conducted with the knowledge of the U.S. Food and Drug Administration.