Archive for November 2017
Nutra Labs Inc. Issues Voluntary Nationwide Recall of Dietary Supplements Bull and Chao Jimengnan Tablets Due to Undeclared Active Pharmaceutical Ingredients
This is a reprint of an FDA Alert. Nutra Labs Inc. is voluntarily recalling lots sold by their firm of the male enhancement supplements Bull 1800 mg Capsules with the production date of 05/08/2016, and Chao Jimengnan 150 mg Tablets with Lot # 20151018 to the consumer level. FDA analysis found the products to be…
[...]Guest View: Drugs from foreign supply chains threaten Illinois patients’ safety
John Redmond is a former FDA official. He has more than 28 years of federal law enforcement experience, ending his law enforcement career as the Special Agent in Charge of FDA’s Chicago Field Office. In this op-ed in The State Journal-Register, he warns that drug importation will expose Americans to dangerous counterfeit medicines and illegal drugs…
[...]Costa Rican Fake Online Pharmacist Pleads Guilty in U.S. Court
Ramiro Navarro Quesada, who was indicted in 2015, and extradited to the United States in August has pleaded guilty to charges he was running fake online pharmacies, according to a Department of Justice (DOJ) press release.
[...]Washington Naturopath Sentenced To Jail In Misbranded Drug Case
Washington state naturopathy Rick Marschall was convicted in 2011 for illegally prescribing misbranded drugs to his patients. Sentenced to probation, he kept prescribing causing the state to suspend his license in 2013. He continued to practice medicine without a license and just received a jail sentence for prescribing the same misbranded drugs to his patients…
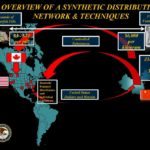
[...]China Smuggling Case Illustrates the Role Canada Plays Bringing Fentanyl into United States
The recent indictment of six Canadians demonstrates the role Canada played as part of a larger international fentanyl smuggling ring that shipped fentanyl compounds to the United States.
[...]Limbrel Capsules by Primus Pharmaceuticals: FDA Advisory – Linked to Potentially Life-Threatening Health Problems
In total, the FDA has received 194 adverse event reports regarding Limbrel, of those, 57 of the cases contained sufficient information to analyze in detail whether Limbrel was associated with an adverse event; 30 of these contained sufficient information to use the Council for International Organizations of Medical Sciences (CIOMS) causality assessment method to determine the likelihood that an association between the consumption of Limbrel and the adverse events reported exists.
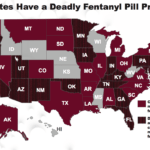
[...]Law Enforcement and Medical Communities Fear that Fake Meds Made with Fentanyl Are Flooding U.S. Streets
Counterfeit pills made with fentanyl have flooded the streets of America. No one know how many people have been hurt or killed, but law enforcement and medical communities are gravely concerned…
[...]Woman Receives 20,000 Fake Pills Instead of Yoga Mat in Mail
In October, a woman in Rock Hill, South Carolina received a package from Walmart that she assumed was a yoga mat she had ordered. Instead, the package contained over 20,000 oxycodone pills.
[...]The ‘Price Savings’ of Drug Importation— Unboxing the Myth
Wayne Winegarden and Nouran Ghana’s editorial was published in Inside Sources on November 15, 2017. In it, they take a hard look at the supposed “price savings” of drug importation and find that the promises do not live up to what would happen. They believe that Americans deserve a better solution than plundering the drug supply of a neighboring country…
[...]FDA-OCI Increasing Efforts To Keep Counterfeit Drugs From Getting Into The Country
FDA Commissioner Scott Gottlieb spoke with FDA-OCI agents about the importance of their work and how the agency is increasing staff at International Mail Facilities and ports of entry to help identify and keep fake drugs and illicit opioids out of the country…
[...]