The NABP reports that illegal actors are selling desperate patients substandard and counterfeit versions of the new generation of weight loss medicine.
Read MoreSTOP CLOPEZ CORP is voluntarily recalling one lot of Schwinnng capsules to the consumer level. FDA analysis has found the Schwinnng products to contain Nortadalafil. Nortadalafil is an active drug ingredient known for the treatment of male erectile dysfunction. The presence of Nortadalafil in Schwinnng capsules makes it an unapproved new drug for which the safety and efficacy have not been established and, therefore subject to recall.
Read MoreThe Southern District of New York sentenced criminals from two separate HIV drug diversion rings.
Read MoreThe products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
Read MorePSM asks major online pharmacy sales platforms for information Today, the Partnership for Safe Medicines wrote to the leading online platforms that enable pharmacy-to-pharmacy drug sales to address a potential danger to the safety of the drug supply. PSM is concerned that counterfeit and diverted medication might be sold on online pharmacy-to-pharmacy platforms by criminals…
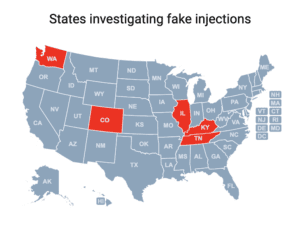
Read MoreCases of a botulism-like illness linked to cosmetic injections have been reported in Colorado, Illinois, Kentucky, Tennessee and Washington.
Read MoreThe state of Florida provided these FOIA-responsive documents to PSM for the state’s final and ultimately approved application to establish a Canadian drug importation program.
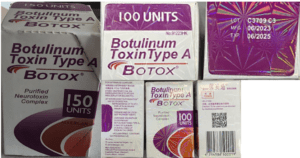
Read MorePeople in nine states sought medical treatment and some were hospitalized after receiving injections of fake Botox in what the CDC has characterized as “non-medical settings.”
Read MoreSome employers are “saving money” on health insurance by hiring vendors to broker personal drug importation between their employees and unlicensed, illegal foreign pharmacies. Read more to understand the dangers of this cost-cutting practice.
Read MoreNumbing creams that exceed the FDA-approved dose can cause irregular heartbeat, seizures and breathing difficulties. CBP has added FDA-regulated products to a pilot program to improve product traceability. Additional domestic and international news about medicine counterfeiting
Read More