This is a reprint of an FDA Alert. STOP CLOPEZ CORP Issues Voluntary Worldwide Recall of Schwinnng Capsules Due to the Presence of Undeclared Nortadalafil When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the…
Read MoreThe Southern District of New York sentenced criminals from two separate HIV drug diversion rings.
Read MoreThe products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
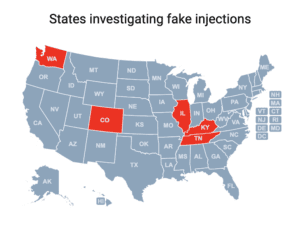
Read MoreCases of a botulism-like illness linked to cosmetic injections have been reported in Colorado, Illinois, Kentucky, Tennessee and Washington.
Read MoreThe state of Florida provided these FOIA-responsive documents to PSM for the state’s final and ultimately approved application to establish a Canadian drug importation program.
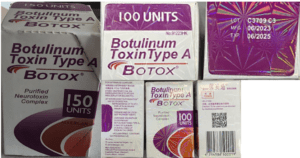
Read MorePeople in nine states sought medical treatment and some were hospitalized after receiving injections of fake Botox in what the CDC has characterized as “non-medical settings.”
Read MoreSome employers are “saving money” on health insurance by hiring vendors to broker personal drug importation between their employees and unlicensed, illegal foreign pharmacies. Read more to understand the dangers of this cost-cutting practice.
Read MoreNumbing creams that exceed the FDA-approved dose can cause irregular heartbeat, seizures and breathing difficulties. CBP has added FDA-regulated products to a pilot program to improve product traceability. Additional domestic and international news about medicine counterfeiting
Read MoreState Senator Scott Wiener’s bill would eliminate under-reimbursement practices that create a dangerous opportunity for criminals to enter the legitimate supply chain.
Read MoreA Las Vegas man was sentenced for money laundering as well as the illegal importation and sale of prescription opioids. Additional news across the U.S. and in India.
Read More