Drug Importation Is Not A New Idea, And It Is Not A Good One, Either
More Than A Decade of Experience Has Shown It Is Dangerous
When an American living in Canada died of heavy metal poisoning in December 2006, investigators found that she had been taking contaminated medicine she had purchased from a reputable-looking online pharmacy that wasn't licensed in the U.S. or Canada.
Others have also suffered injury and death from the drugs unlicensed online pharmacies have sold them. Even when black market products don’t poison patients, they are often not the strength they claim to be. This leads to under- or over-treatment for the patients who take them, as well as drug resistance that makes antibiotics and other treatments ineffective across many patients.
Since 2012, the FDA has been prosecuting unlicensed foreign drug distributors who sold unregulated and sometimes counterfeit treatments to U.S. medical practices, including temperature-sensitive drugs that were compromised by heat, Botox that sent patients to the emergency room, and cancer medicine with no active ingredients. Families of patients like Betty Hunter, who were treated with this cancer medication, will never know whether fake treatments shortened their lives.

Marcia Bergeron died because her medicine contained heavy metals. Photo: National Review of Medicine
“Those people ordered from bad pharmacies. If someone vetted them wouldn’t it be safe?”
Government has not been able to safely administer importation programs.
Illinois devoted much funding in the mid-2000s to setting up drug importation via a list of safe overseas pharmacies, but the program was labor intensive, and it ran into significant safety issues. A 2006 audit revealed that 40% of required inspections of the foreign pharmacies were never completed, and patients were ordering medicine from online pharmacies that had not been approved. Worse, Illinois had no jurisdiction to enforce pharmacy standards over foreign pharmacies. If patients received bad medicine, the state could not protect them.
Any similar government effort to establish safe pharmacies or distributors would take tremendous resources while compromising safety. Four previous U.S. Food and Drug Administration commissioners have studied this problem, and agree.

Read Illinois' audit report of their own importation program.
Independent pharmacy inspectors are not accountable to anyone but themselves.
Companies like PharmacyChecker are for-profit ventures that operate outside regulatory structures. There is no way to trust their inspection process. There is no legal recourse for an American citizen if they are injured by counterfeit or substandard medicines purchased from an online pharmacy, whether or not an independent pharmacy inspector has certified it. Online pharmacies pay PharmacyChecker to be listed as approved on their site. Their customers are online pharmacies, not patients. Protecting the companies they proclaim safe is more important to them than patient safety.
In 2015, for example, the Department of Justice unsealed an indictment against CanadaDrugs.com, an online pharmacy certified by PharmacyChecker, for selling counterfeit and non-FDA approved cancer medications to American medical practices.
According to the indictment, Ram Kamath, then PharmacyChecker’s Director of Pharmacy Policy and International Verifications hid fake Avastin in his garage for CanadaDrugs.com subsidiaries until they could send it back to the United Kingdom. The same month, Kamath wrote a glowing inspection report for CanadaDrugs.com’s operations in Barbados, claiming that the company could ensure the quality of the drugs they sold. The company continued to list CanadaDrugs.com as a recommended site until it could no longer legitimately pretend to look the other direction: when the internet pharmacy and its CEO signed plea agreements in 2018.
Sadly, CanadaDrugs.com is not an isolated example. In a March 2017 blog post, LegitScript listed nine other cases of “Canadian” pharmacies certified by PharmacyChecker or the Canadian International Pharmacy Association (CIPA) that had been prosecuted for dispensing drugs—including controlled substances—without prescriptions, for misrepresenting the source of their medicines, or for selling counterfeit medication.
“Don’t be ridiculous. The Canadian drug supply is safe. We can take their word for it.”
When Americans order medicine from “Canadian” pharmacies, they are not receiving the same medicine Canadians pick up at their pharmacies.
For one thing, Canadian pharmacists cannot legally dispense Health Canada-approved medicine to Americans without a Canadian prescription, and co-signing American prescriptions without a patient exam violates standards of care in every Canadian province.
For another, Canada doesn't have enough medicine to supply Americans. The U.S. has nine times the population of Canada, and if even 20% of U.S. residents started purchasing their medications from the Canadian drug supply, we would use up their supply of prescription drugs in just five months. In fact, Canada already experiences drug shortages.
Instead of depleting the Canadian drug supply, Canadian online pharmacies sell Americans medicine from countries like China, Turkey or India. Black market drug sellers ship their products through multiple countries to evade regulation, sometimes stopping in Canada, sometimes shipping directly to American patients. Criminals even falsify labels and forms to persuade Americans that this medicine is actually Canadian.
Because Canadian online pharmacies aren't selling their drugs to Canadians, Health Canada does not regulate or inspect the products they sell, and actually warns Canadian citizens not to buy from unlicensed online pharmacies. Because the FDA is also unable to inspect these drugs, no one is checking them. Without oversight, Canadian online pharmacies operate as pass-through organizations and there is no way to ensure that they are selling Americans safe and effective medicine.
“Doesn’t the threat of jail deter these businesses?”
No. When a drug counterfeiter overseas sells American citizens fake or substandard medicine, our hardworking law enforcement must rely on international goodwill to extradite them. Criminals in foreign countries are difficult to extradite for prosecution, and when the Department of Justice can prosecute them, they often receive light sentences.
When CanadaDrugs.com pleaded guilty to selling American medical practices $78 million in non-FDA approved drugs, they signed a deal that led to $34 million in fines and forfeitures. Their CEO Kris Thorkelson agreed to a six-month house arrest, four and a half years of probation and a small fine. Six additional individuals had all charges against them dismissed. No one served any time in prison.
In August 2018, the Justice Department indicted two Shanghai residents for allegedly trafficking drugs--including counterfeit Adderall--that led to the death of two people in Ohio. China and the U.S. do not have an extradition treaty, however, and China has refused to send its citizens to the U.S. to be prosecuted in the past. These drug counterfeiters may never even see a courtroom.
Why Do Policymakers Keep Saying Importation Will Make The Opioid Crisis Worse And Endanger Law Enforcement?
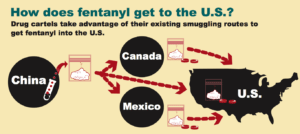
Over 29,000 Americans overdosed on synthetic opioids such as fentanyl in 2017, most of which is imported from Chinese labs by global drug smugglers, including drug cartels such as the Sinaloa cartel. As October 2018, fentanyl-based counterfeit medications had been found in 45 states and killed Americans in at least 27 states.
Read Fentanyl 101 to learn more.
“I’ve heard all this. What does this have to do with drug importation?”
Fentanyl is coming the same way imported medicine will come—through the United States Postal Services’ International Mail Facilities (IMFs). In 2017, IMFs processed 275 million packages--up 232% since 2013, but they only had the capacity to screen 10,200 suspicious packages within those 275 million. 86% of the packages they were able to screen contained black market drugs.
Importation will increase the volume of drugs shipped from overseas, and an even smaller percentage of packages will be inspected. More fentanyl and more counterfeit drugs will inevitably slip through.
A tidal wave of imported medicine will overwhelm our systems and put Americans at greater risk.
“Can’t we just track imported medicine like we track our own?”
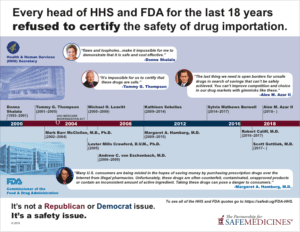
Read statements from the heads of HHS and FDA against allowing drug importation
No. In 2013, Congress passed the Drug Quality and Security Act of 2013, which set in motion a multi-year, extremely complex implementation of supply chain safety measures that will make our closed drug supply even safer than it is now.
There is no way to ensure that medicine imported from unregulated foreign entities complies with new regulations. Policymakers have explained that importation will break this “track and trace” system and FDA Commissioners from both Republican and Democratic administrations agree.
Canadians think that Americans ordering medication from Canada is a bad idea.
The list of issues with Americans ordering from the Canadian drug supply is legion.
First, it's unlikely we can actually get medicines out of their domestic drug supply. Canada does not prosecute people who put up fake Canadian web pharmacies so those sites abound online. But if we actually did try to get Canadian domestic drug supply, it's not even legal for their pharmacists to dispense to us. Three different Canadian pharmacy regulators recently said this is not allowed under Canadian licensing.
Canada doesn't have enough medicine supply to start filling our prescriptions. PSM's board member Marv Shepherd researched this and estimated that if 20% of U.S. residents started purchasing their medications from the Canadian drug supply, they would strip Canada's name brand drug supply in 201 days.
These same concerns were also brought up in recent letters from Canadian patient groups as well as pharmacy regulators from the Canadian provinces of Manitoba and Newfoundland and Labrador. Even the association that represents pharmacy boards all across Canada weighed in on the fact that this is illegal.
