News Coverage
The Partnership for Safe Medicines has been publishing information about the counterfeit drug problem around the world for more than a decade. With experts leading the organization and a committed and passionate set of writers and editors, our content is more in-depth than many other sources, which simply copy links to the news from other websites.
The Southern District of New York sentenced criminals from two separate HIV drug diversion rings.
The products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
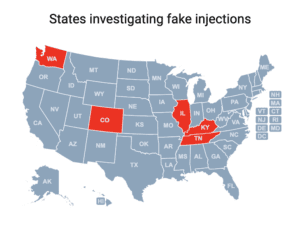
Cases of a botulism-like illness linked to cosmetic injections have been reported in Colorado, Illinois, Kentucky, Tennessee and Washington.
The state of Florida provided these FOIA-responsive documents to PSM for the state’s final and ultimately approved application to establish a Canadian drug importation program.
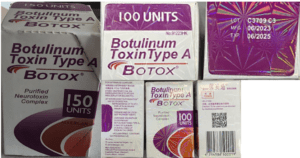
People in nine states sought medical treatment and some were hospitalized after receiving injections of fake Botox in what the CDC has characterized as “non-medical settings.”
Some employers are “saving money” on health insurance by hiring vendors to broker personal drug importation between their employees and unlicensed, illegal foreign pharmacies. Read more to understand the dangers of this cost-cutting practice.
Numbing creams that exceed the FDA-approved dose can cause irregular heartbeat, seizures and breathing difficulties. CBP has added FDA-regulated products to a pilot program to improve product traceability. Additional domestic and international news about medicine counterfeiting
State Senator Scott Wiener’s bill would eliminate under-reimbursement practices that create a dangerous opportunity for criminals to enter the legitimate supply chain.
A Las Vegas man was sentenced for money laundering as well as the illegal importation and sale of prescription opioids. Additional news across the U.S. and in India.
The Indian press reported that a Delhi-based ring sold fake cancer medicines in the U.S., China, and India. U.S. embassies and consulates in Mexico warned spring break travelers about counterfeit medicine. More domestic and international news about counterfeit medicines.
In spite of Etsy’s terms of use, there is a thriving trade in chemicals on the site, including those sold to individuals seeking homemade versions of highly regulated pharmaceuticals.
Colorado’s amended importation plan cuts prospective drugs by 78%. FDA Commissioner Califf warned about counterfeit medicines. A British couple who allegedly provided U.S. clinics they owned with illegally imported cancer medicines is facing extradition. Counterfeit medicine in Canada, Mexico and elsewhere.
Colorado’s updated Canadian drug importation application to the FDA cuts the drug list from 112 to 24 and adds Ozempic to the list of medicines it wants from Canadians.
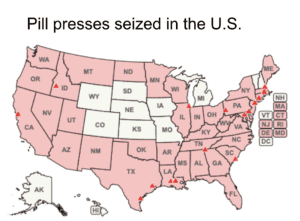
The DEA put all e-commerce platforms on notice of their obligations whenever they facilitate transactions involving pill presses.
A Maryland drug distributor lost $2.7 million in a settlement over selling secondhand medicine to U.S. pharmacies. Additional news in Alabama, California, New Jersey, Tennessee and more.
Companies in New York and Florida were selling unapproved GLP-1 agonists. Operation Shield IV removed $69 million in illicit medicine and doping substances from European markets. A California man who distributed smuggled tianeptine received a two-year sentence.
PSM opposes the practice of reimbursing pharmacies below the cost of acquisition of medicine and supports efforts to reform reimbursement systems that eliminate this market dynamic that threatens the safety of our drug supply.
The government won prosecutions in Massachusetts and New Hampshire against defendants selling non-FDA approved drugs. A family warns about tianeptine. Pill presses in Texas, Virginia and West Virginia.
“We are deeply concerned about the impact importation of unapproved foreign prescription drugs will have on counterfeit medication in the United States, especially as FDA considers approving additional SIPs.”
DoJ alleged that eBay did not keep and submit required records to the DEA. Snap’s CEO spoke in support of the Cooper Davis Act. The FDA warned about copycat eye drops. Additional domestic and international news about fake and substandard medicine.
PBMs, by under reimbursing pharmacies, are creating a demand for pharmacies to seek lower priced medications even when they can’t exist at that price.. Criminals appear to be happy to become part of the supply chain.
The state of Maine opened it’s border to Canadian drug importation program for Maine residents. Dr. Kenneth McCall purchased and tested some of the medicines being advertised and sold to Maine residents and found them lacking in safety. Listen to his story.
PSM’s Shabbir Imber Safdar sat down with three law enforcement experts about the problems they are seeing with counterfeit medicine. Watch here.
An explainer for the $59mm settlement between eBay and the Department of Justice over pill press sales. What does this mean for the problem of illegal pill presses? How is eBay responsible for what a buyer and a seller do on its platform?
PSM’s Shabbir Imber Safdar speaks to Canadian patient advocate Durhane Wong-Rieger about the drug shortage situation in Canada.
What drugs have states said they plan to export from Canada’s drug supply? We combed all their applications to the FDA and compiled that list into one document.
Family advocates Andrea Thomas and Amy Neville will distribute naloxone at the Dirksen Senate Office Building. The VA will use the NABP’s digital DSCSA platform to help with compliance. Guilty pleas for dark web drug trafficking and manufacturing fake oxycodone, and more.
On January 17, 2024 PSM wrote the Senate Subcommittee on Intellectual Property to endorse S.2934, which would expand the Trademark Act of 1946 to extend liability for selling harmful counterfeits to online platforms. Read the letter here.
Tests of the counterfeits showed that they were made of many substances, including acetaminophen, duloxetine, caffeine and methamphetamine. Overseas, authorities seized counterfeit pregabalin in Belfast. Pakistan recalled toxic cough syrup. Fake Xanax trafficker Ryan Farace got an additional sentence for money laundering. News about pill presses in five states.
PSM recently published contracts, some obtained via FOIA, with key vendors implementing Colorado’s and Florida’s Canadian drug importation plan. Read them here.
Florida’s October 20, 2023 SIP application is the second to last application before FDA approval. The final application was filed on November 16, 2023.
Homeland Security seized over 1,800 pill presses in October and November 2023. The FDA warned Amazon not to sell supplements made with undeclared pharmaceuticals and issued another warning about toxic yellow oleander. More domestic and international news about counterfeit medicine.
PSM’s statement on the FDA decision and full coverage including media stories, official documents, and statements from stakeholders.
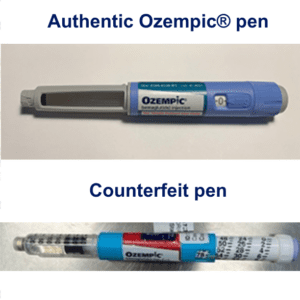
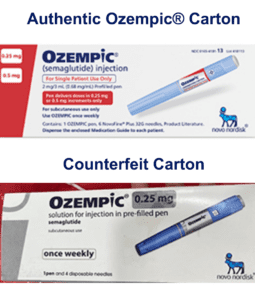
FDA continues to investigate counterfeit Ozempic (semaglutide) injection 1 milligram (mg) in the legitimate U.S. drug supply chain and has seized thousands of units of the product. FDA is aware of five adverse events from this lot.
This is a reprint of an FDA Alert. 8th Avenue Pharmacy Issues Voluntary Nationwide Recall of Notoginseng Formula Special Gout Granule Due to the Presence of Hidden Drug Ingredients, Diclofenac and Dexamethasone When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does…
Overdose deaths among Americans 65 and older quadrupled over the last 20 years. A Brooklyn man pleaded guilty to selling second hand HIV drugs to local pharmacies. The FDA explained “What You Should Know about Eye Drops.” More domestic and global news about counterfeits.
A CNBC story covered details about Lazaro Hernandez’s $230 million HIV drug diversion scheme. A recent survey showed that Americans don’t understand online pharmacy safety. More news about counterfeit medicines across the globe.
Neptune’s Fix tianeptine products have caused fainting and seizures. A study in California found unlisted Viagra or Cialis in 67% of over-the-counter sexual performance supplements. Additional news about fake oxycodone in Mexican pharmacies and other counterfeit medicines in the U.S. and overseas.
It isn’t just U.S. regulators that warn against ordering medicines from unlicensed pharmacies. Canadian regulators have the same concerns about the safety of unapproved medical products.
Canadian criminal gangs have sold illicit fentanyl in the U.S., Australia, New Zealand and Japan. Costa Rica shut down a domestic fentanyl pill operation. More news in the Kenya, Nigeria, Sri Lanka, and the U.K.
China agreed to cooperate with the U.S. in stopping pill press and precursor sales. An Indian company launched a U.S. recall of contaminated eye drops. Counterfeit medicine news overseas and in the U.S.
In September, the Partnership for Safe Medicines and others filed a supplement to our 2021 Citizen’s Petition asking the U.S. Department of Health and Human Services not to authorize Florida’s Canadian Drug Importation Plan.
As of September 2023, at least 3 Americans have been hospitalized because of fake semaglutide products. The Treasury Department sanctioned more people and businesses it says are trafficking fentanyl into the U.S. More news in the EU, Nigeria and three U.S. states.
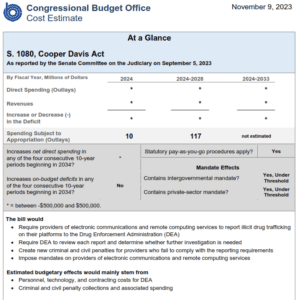
For those following the Cooper Davis Act (S.1080) which would require social media platforms to report drug dealing when they discover it, the Congressional Budget Office has analyzed the legislation and said it will “not significantly increase net direct spending” and “not increase on-budget deficits”.
Interpol announced the results of Operation Pangea and U.S. CBP reported on pill press seizures. News about counterfeit medicine in the U.S, Belgium, China, and Indonesia,
FDA warned about contamination in 26 different over-the-counter eye drops. Ten New York residents were indicted in another black market HIV medicine scheme. Additional stories in California, Florida, and Texas.
Risk Statement: Consumption of undeclared diclofenac could result in serious adverse events that include cardiovascular, gastrointestinal, renal, and anaphylaxis in patients taking concomitant NSAIDs and/or anticoagulants, such as Warfarin, in those who have allergies to diclofenac, or those with underlying cardiovascular, gastrointestinal, renal, and hepatic illnesses.
A delay in Florida’s response to FDA questions means the agency’s decision won’t be by October 31 after all.
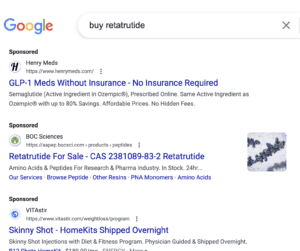
Sketchy online vendors are selling “retatrutide” even though it’s still in clinical trials. A man who sold U.S. pharmacies secondhand prescription medicines was sentenced. Domestic and international stories about fake drugs.
Almost 200 packages of fake Ozempic traveled from Austria to the U.K. via Germany. An 11th man was sentenced in a $52mm cough medicine counterfeiting scheme. Europol reports the seizure of medical products. Additional news about pill presses in Mexico and the U.S.
The leaders of the “Genesis II Church of Health and Healing” received a cumulative 34 years in prison. A NYC pharmacist pleaded guilty to fraud and money laundering after selling black market HIV drugs. Pill press operations busted in NYC and Rhode Island.
The FDA is cracking down on lax safety testing of raw drug ingredients. Major players in HIV drug and cough syrup counterfeiting schemes pleaded guilty in federal court.
Counterfeit Avastin in Pakistan blinded over 70 retinopathy patients.
CBP at JFK Airport intercepted 14 pairs of pill press dies. Additional news about illicit pill presses in five states.
Shabbir Imber Safdar, executive director of the Partnership for Safe Medicines, will participate in the upcoming United States Patent and Trademark Office Roundtable: Future strategies in anti-counterfeiting and anti-piracy.
USFDA is concerned that eight companies, including two pharmacies, are illegally marketing unapproved ophthalmic drug products that pose a heightened risk to users. Counterfeit pills make the DHS’ threat assessment list for 2024.
Data recently shared in the Morbidity and Mortality Weekly Report highlights a growing problem: Counterfeit pill availability is rising in the drug supply chain, but also the illicit drug market.
Running slides between conference sessions and during breaks is a great way to fit in a little extra information. Use these slides to teach your community about counterfeit medicines in the U.S.
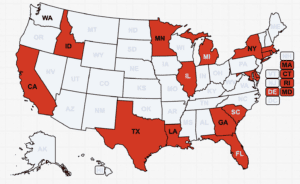
A U.K. couple was sentenced for selling 65 different brands of unapproved medicines. Counterfeit therapeutic medicines reported in India, Turkey and Pakistan. News about counterfeit pills made of dangerous substances in 13 states
CDC data shows an alarming climb in pill deaths, particularly in western states. Narcan, which reverses opioid overdoses, is now available over-the-counter. Fake medicine seized in Las Vegas. News involving counterfeit pills in 17 states.
The FDA asked Florida to clarify its drug importation plan. More contaminated eye drops in the U.S. Fake medicine, including a cancer treatment, in Mexico. U.S. patients treated with illegally imported Botox and lip filler. News about counterfeit pills made with fentanyl in 18 states.
On August 3, the Partnership for Safe Medicines submitted comment on the U.S. Patent and Trademark Office’s Future Strategies in Anticounterfeiting and Antipiracy.
Poisonous “Nuez de la India” made with yellow oleander. More about black market weight loss medicine. Federal authorities arrested a fugitive for selling fake COVID preventatives. News about counterfeit pills in 24 states.
On August 16, 2023 Executive Director Shabbir Safdar asked senior FDA officials to require wholesalers to provide pharmacists with complete transaction logs for every medicine they dispense until the track-and-trace system is fully online.
Hospitals are being impacted by drug shortages. More U.S. sanctions against Sinaloa Cartel members. Uzbeki courts pursue the fake cough syrup supply chain. News about counterfeit pills in 15 states.
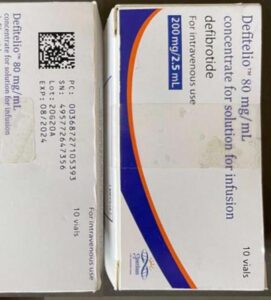
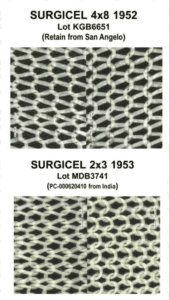
A lawsuit over fake Surgicel hemostat concluded. New research spotlights fraud in sports supplements. A recently jailed cough syrup counterfeiter also settled allegations of violating the Controlled Substances Act. Fake medicines reported in Canada, Egypt, India, and Mexico.
Federal judges sentenced two men who ran a fentanyl ring from a Canadian prison, a pharmaceutical exec who trafficked fake cough syrup, and a black market drug trafficker. Additional stories about counterfeit pills and related casualties in 21 states.
Pill presses prohibited in Washington state. WHO issued an alert about counterfeit cough syrup. Jury convicted four in Florida who peddled bogus COVID-19 cure. Prosecutions in 11 states involved the trafficking of counterfeit pills and related deaths.
Legislation moves forward in the Senate for social media platforms to report dealers to the DEA. A Shanghai resident sentenced for selling counterfeit cat medicine. Missouri resident sentenced for injecting clients with non-FDA-approved Botox and fillers, hurting multiple people. Stories of counterfeit pills in 17 states.
S.1080, which requires big tech platforms to report drug dealers they discover to the DEA, passed out of the Judiciary committee and is headed to the Senate floor.
Global coalition launched to combat synthetic drugs. One-third of packages examined in Operation Broader Sword contained unapproved medicines, medical devices, or precursor chemicals. Prosecutions in seven states involved the trafficking of fentanyl pills and related deaths.
The FDA warned Safe Chain Solutions for trading with unlicensed sellers. Other black market HIV drug cases are moving forward. The CDC reported a spike in fentanyl/xylazine deaths. Authorities seized a pill press, smuggled codeine and millions of counterfeit pills made of fentanyl. Prosecutions involved the trafficking of and deaths related to fentanyl pills in 10 states.
In this editorial, which appeared in the Corpus Christi Caller on June 30, 2023, Doctor of Pharmacy Kenneth McCall shares his negative experience with imported medicines.
“On April 20, 2022, FDA warned consumers not to purchase or use “Artri Ajo King” as FDA has received adverse event reports, including of liver toxicity and death, associated with the use of “Artri Ajo King” and similarly named products. While the Agency has not sampled and tested this product from your inventory to date, be advised of our serious concern about the safety of these products and that it is your legal responsibility under federal law to ensure this product does not contain any undeclared and potentially harmful ingredients.”
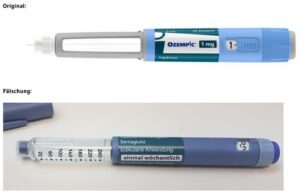
Fake Ozempic has been found in the U.S. The leader of an HIV drug counterfeiting ring got 15 years. The Justice Department indicted Chinese companies and arrested two executives who allegedly sold fentanyl precursors into the U.S. Tainted cough syrup reached at least 12 countries. Stories about counterfeit pills in 20 states.
On June 16, 2023 Novo Nordisk warned that a fake version of the diabetes drug Ozempic had been found in the U.S. The injection pen, which contained insulin instead of semaglutide, was reportedly purchased at a retail pharmacy. It’s one of a flurry of incidents involving Ozempic and its sister drugs.
More than 50% of the pills investigators bought in Mexican pharmacies were fake–and many contained illicit drugs. COFEPRIS warned about fake immunosuppressants and anemia drugs. A drug dealer who targeted Carrollton, Texas teens pleaded guilty. More news about pills seizures and prosecutions in 20 states.
When medical licensing boards take up cases of misconduct, they face some real challenges because criminal cases resolved without trials may leave almost no public evidence. Documenting evidence of wrongdoing in sentencing memorandums helps licensing boards, and also patient victims, to see the full scope of a case.
The FDA increased facility inspections 44% in 2022 and prevented 222 drug shortages. Men in Colorado and Virginia were sentenced for distributing unapproved prescription drugs. News about drug seizures and prosecutions trafficking counterfeit pills in 22 states.
William Merlino, whose weight loss products killed a U.K. man, will serve almost 3 years in prison. The U.S. imposed sanctions on pill press makers in China and New Mexico. Regulators found more fake semaglutide—this time in Australia. Prosecutions and pill and pill press seizures in 24 states.
North Carolina made using a pill press to make counterfeit controlled pills a felony. Black market and fake medicines were reported in Canada and Mexico. More news involved counterfeit or illegally imported pills in 22 states.
With the passage of HB 25 in the Texas State Senate and Governor Abbott’s impending signature, Texas is poised to become the seventh state in the U.S. to attempt to create a program to import medicine from Canada. This policy has failed in six other states, cost taxpayers millions of dollars, and drawn opposition from the Canadian government.
Grieving families continue to mourn the ones they lost to dubious and counterfeit medicine. Three men were convicted of manufacturing and selling counterfeit pills in the U.K. Additional news involving counterfeit pills made with fentanyl in twenty states.
Families across the country observed Fentanyl Awareness Day. Hidden pharmaceuticals in arthritis “supplements.” Fake medicine in Ireland, India and Australia. Additional news involved counterfeit pills made with fentanyl in 17 states.
The FDA has issued consumer warnings on three different dietary supplement sold as herbal compounds intended to treat joint pain, rheumatoid arthritis, gout, and liver detoxification. Each product contains undeclared prescription drug ingredients.
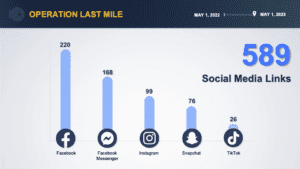
Federal agencies report the results of Operations Last Mile and SpecTor. Fake medicines in Mexico, Nigeria and the Philippines. Pill press seizures in Texas and West Virginia. News involving counterfeit fentanyl or meth pills in 19 states.
FOR IMMEDIATE RELEASE – April 26, 2023– West Sacramento, CA, Gear Isle is voluntarily recalling the following products listed in the table below to the consumer level. FDA analysis has found the products to be tainted with prescription drug ingredients.
TruVision Health LLC is recalling the dietary supplement products listed below because they contain the unapproved dietary ingredients hordenine and/or octodrine/DMHA (1,5-Dimethylhexylamine).
Canada resists the U.S. pilfering its drug supply. Officials warn about semaglutide sales on social media, a dangerous appetite supplement and black market cosmetic injectables. A California doctor will pay over a million for treating patients with illegally purchased drugs. More reports of tainted cough syrup, and news about fake pills made of fentanyl and other drugs in 20 states.
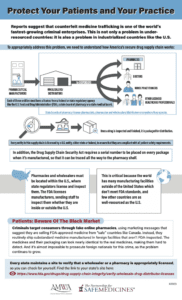
A new infographic created by the Partnership for Safe Medicines and the American Medical Women’s Association educates healthcare professionals on the importance of only sourcing drugs from within the secure U.S. drug supply chain…
This week: A California man is among those killed by counterfeit pills at Mexican pharmacies. A 6th lawsuit was filed over pill dealers on Snapchat. Fake cancer treatments reported in the UAE, Kyrgyzstan and India. A new counterfeit drug bust in the U.K. Additional news involving counterfeit painkillers and fake Adderall in over 20 states.
Amoxicillin shortages underscore the need to buy from licensed U.S. pharmacies. The Justice Department indicted Sinaloa cartel members. Xylazine mixed with fentanyl is an emergent threat. Another Canadian is being tried for pill trafficking. News about prosecutions, seizures and deaths relating to counterfeit prescription pills in 24 states.
A Canadian doctor working in the U.S. will not be allowed to prescribe Canadian drugs. Washington legislators passed a law to regulate misuse of pill presses. Fake Xanax cases conclude in Wisconsin and Virginia. Crackdown on drug counterfeits in India. Mexican regulators warned about more fake drugs. News involving counterfeit pills in 23 states and the U.K..
The FDA approved over-the-counter Narcan. Congress is crafting legislation to regulate xylazine. A police union executive allegedly imported a fentanyl analogue and prescription pills containing controlled substances. Prosecutions, pill seizures and deaths in 16 U.S. states.
PSM provided our thoughts to the US Senate Health, Education, Labor, and Pensions committee when they requested input on the authorization of the Pandemic All Hazards Preparedness Act.
The DEA warned about a sharp increase in the trafficking of fentanyl mixed with xylazine. The U.S. FDA warned to a supplement manufacturer and a website selling ivermectin to stop selling unapproved drugs. A Montana man who hid his pill press operation under a haystack has been sentenced to 16 years in prison. More news in 15 U.S. states.
The “Cooper Davis Act” would require social media platforms to report users of social media found to be selling controlled substances on their platforms to the Drug Enforcement Administration.
H.B. 25 would require Texas’ Health and Human Services Commission to design a program for bulk importing prescription medicines under 21 USC 384 of the U.S. Food, Drug, and Cosmetics Act, more commonly known as a Section 804 State Importation Program (SIP). Below, we outline the many reasons this proposal is unsafe and unworkable.
Federal legislators reintroduced the CAST Act, which would strengthen penalties for using pill presses to make fake medicines. Health Canada warned parents not to buy unapproved cough syrups advertised on Facebook. Mexican soldiers seized almost two million fentanyl pills in Tijuana. News about prosecutions, pill seizures and deaths in 21 U.S. states.
This recall has been initiated because the Product is labeled as a dietary supplement that, in the opinion of the government, makes unsubstantiated health claims that the product will prevent, treat, or cure COVID-19.
A company is facing charges for medical device fraud. Mexican regulators shut down pharmacies for selling fake prescription pills made with fentanyl, something that has been happening since at least 2019. International news about contaminated cough syrup, and about prosecutions involving counterfeit pills and fake pill seizures in 20 U.S. states.
On Tuesday, February 28, 2023, the Partnership for Safe Medicines convened pharmacists, law enforcement, policy experts and family advocates to educate legislators about key issues in the fight against counterfeit medicines. Learn more and watch the briefing here.