February 17, 2026: Pennsylvania compounding pharmacy earns another FDA warning letter
Major Stories
A Pennsylvania compounding pharmacy received another FDA warning letter following a series of enforcement actions, including a $1 million state fine in October. The Court of Appeals affirmed a sentence for a manufacturer of counterfeit pills.
Boothwyn Pharmacy, LLC is facing intensified scrutiny after the U.S. Food and Drug Administration issued a warning letter citing sterility failures, subpotent injectable and ophthalmic drugs, and insanitary production conditions at its Pennsylvania facility. The letter follows earlier enforcement actions, including a $1 million state fine for operating in unlicensed spaces and prior deficiencies in compounding and quality-control.
The FDA said that the pharmacy failed sterility and potency testing and documented that some sterile drugs were distributed before final sterility results were confirmed. Inspectors also found subpotent ophthalmic and injectable products, including semaglutide and tirzepatide formulations.
On February 4, 2026, the United States Court of Appeals for the Sixth Circuit affirmed a 90-month sentence in United States v. Wala, upholding a district court’s application of the federal sentencing guidelines in a counterfeit drug prosecution under the Food, Drug, and Cosmetic Act. Omar Wala pleaded guilty to conspiring to manufacture and sell counterfeit generic alprazolam pills in November 2023.
Applying a fraud guideline, the district court calculated a $32 million loss based on the $2 street price paid by end users, and the Sixth Circuit agreed that street-level buyers were the intended victims. The FDA Law Blog wrote that the case is a roadmap for how other federal courts could rely on street value to apply significant sentencing enhancements in black-market counterfeit drug cases.
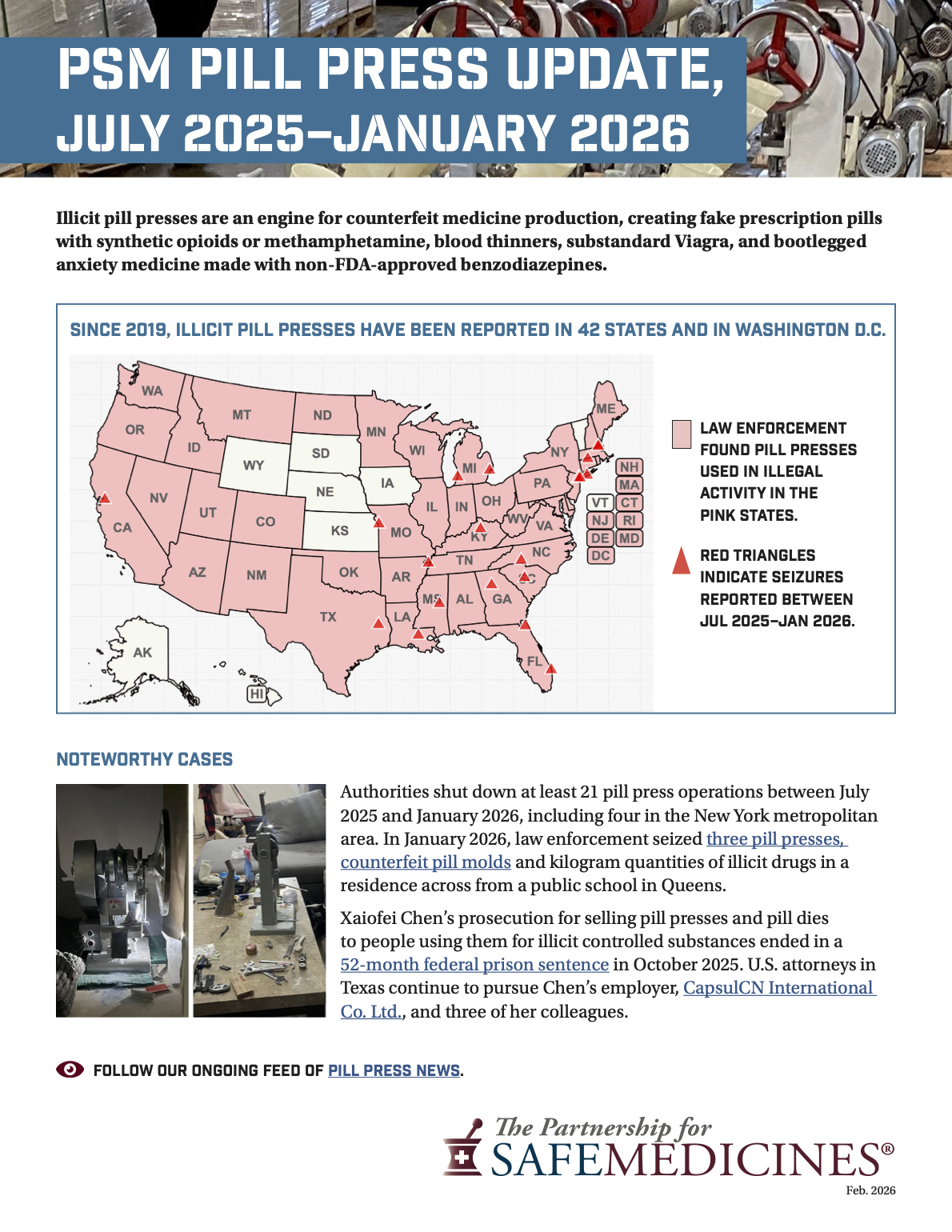
Our latest handout from PSM tracks international and domestic seizures, notable prosecutions, federal and state legislation, and third-party pill press listings, providing a comprehensive resource for policymakers, law enforcement, and public health professionals. Explore the update and access additional resources here.
Domestic
14 arrested in Washington state following a wiretap investigation, and an international prescription referral service advertising the importing of Ozempic from India.
The DEA announced the arrests of 14 individuals for drug and gun trafficking following a wiretap investigation. First Assistant United States Attorney Neil Floyd said, “The defendants in this indictment allegedly engaged in a wide range of criminal conduct – pressing narcotic pills for distribution… [one defendant] distributed the pills he manufactured in western Washington and across the country through the mail. So far, three overdose deaths have been connected to Salgado’s activity.”
The press release alleges that some defendants hacked into drug distribution companies and rerouted shipments of controlled substances to sell locally, while another defendant allegedly paid kickbacks to a pharmacist to fill fake prescriptions for narcotics, including oxycodone. He was arrested picking up a fraudulent prescription of promethazine and codeine. Seven kilograms of counterfeit oxycodone pills have been recovered so far in the investigation.

Press conference in Seattle. (photo: DEA)
A Canada-based website is offering steeply discounted India-sourced Ozempic to U.S. patients despite not being a licensed pharmacy. The company describes its business as an “international prescription referral service” that connects patients to pharmacies outside the United States. Novo Nordisk, the manufacturer of Ozempic, told Reuters it has no relationship with the platform and has not supplied it with product from India, raising questions about how the drug is being obtained.
Two Massachusetts men were arrested and charged after authorities seized two pill presses and thousands of counterfeit pills suspected to contain methamphetamine. Prosecutors allege the pair manufactured and distributed the fake pills, and they face federal drug conspiracy charges carrying up to 20 years in prison.
Eleven alleged members of a drug trafficking organization were indicted in Louisiana on charges of conspiring to distribute large quantities of fentanyl, methamphetamine, and cocaine. Authorities allege the group imported drugs into Louisiana. A related investigation led to the seizure of fentanyl pills and a pill press.
Regulators protecting patients in the news
In a January warning letter to an Arizona facility that produces kids' bubblegum toothpaste, the FDA cited significant CGMP violations, including inadequate controls to prevent cross-contamination from potent ingredients, insufficient cleaning validation, release of drug products without proper laboratory testing, and a lack of effective quality unit oversight.
In another warning letter, a Texas facility was cited for failure to ensure sterility of drug products, inadequate aseptic processing and facility design, lack of process and cleaning validation, and insufficient batch testing before release. The FDA recommended the company remove any batches of a solution it currently distributes and consider ceasing further distribution, noting the firm erroneously claimed these products were not drugs.
Asteria Health, a 503B compounding facility, voluntarily recalled over 118,000 estradiol sterile pellets used in hormone replacement therapy due to potential metal contamination. The FDA classified the recall as Class II, affecting multiple strengths with expiration dates from February to October 2026.
Legislation
Maryland SB837 and HB1440 would prohibit certain insurers and managed care organizations from requiring prior authorization, step therapy, or other coverage restrictions for prescription drugs that have been reviewed by the Prescription Drug Affordability Board.
Oregon HB4040 modifies the PDAB statute so the board “may identify at least one insulin product,” giving it discretion not to identify any insulin.
Massachusetts H5087 establishes licensure requirements and definitions for medical spas, while Arizona HB4047 sets licensure rules for medical spas in that state.
GLP-1s
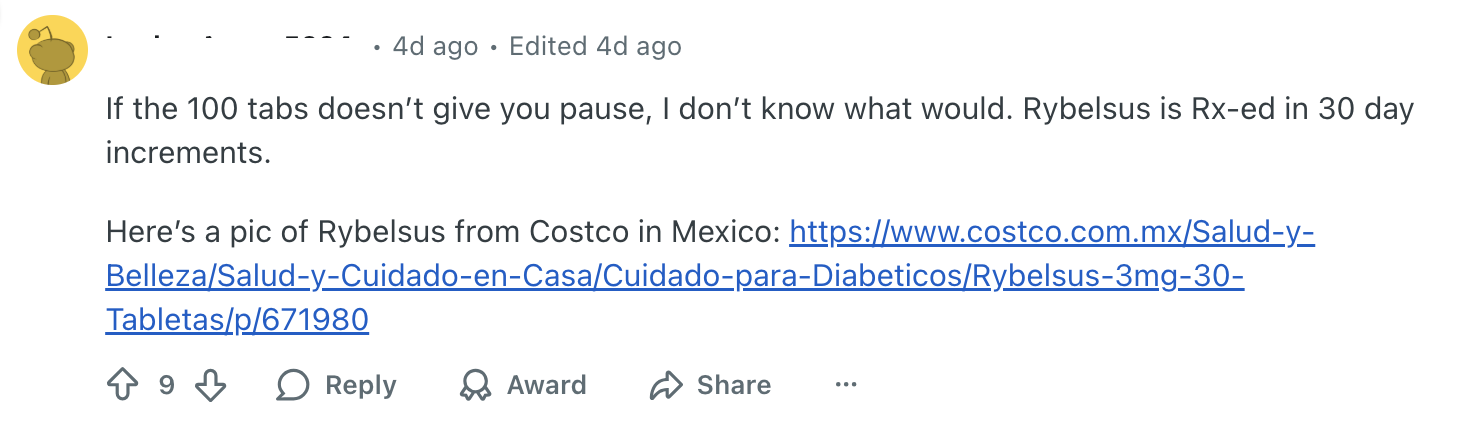
This February 2026 Reddit post features a user expressing doubt about the authenticity of Rybelsus tablets brought back from Mexico by a friend. The user noted that a Google image search failed to produce any matching packaging, and commenters quickly pointed out several red flags, most notably the bottle's "100 tabletas" count, a quantity that contradicts the standard 30-day supply for this prescription medication.
If the poster were using FDA-approved medicine obtained through a licensed pharmacy with a valid prescription, they would not have to crowd-source information to determine if their medication was counterfeit or safe to consume.
International
Three arrested for selling unapproved and potentially counterfeit weight-loss medications in Wales, 20 million illegal erectile dysfunction drugs seized in the U.K., a drug warehouse shut down in Nigeria, and an Indian fake drug factory barred.
Welsh authorities launched an investigation into the sale of illegal and potentially counterfeit weight-loss medications, including retatrutide, tirzepatide, and semaglutide, advertised online and sold at beauty salons. North Wales Police executed warrants at three locations and arrested three on suspicion of fraud.
The MHRA reported the seizure of nearly 20 million illegally traded erectile dysfunction pills in the U.K. over the past five years, including 4.4 million in 2025 alone. The agency warned that buying medicines from unregulated online sources poses serious health risks. Many of the seized pills contained unknown or dangerous doses of active ingredients.
The Delhi Police’s Anti-Narcotics Task Force arrested nine people while uncovering a sophisticated fake drug factory in Gaya, Bihar. The task force seized large quantities of counterfeit medicines, including zinc and azithromycin tablets, paracetamol, and tramadol-based opioids, as well as heavy manufacturing equipment.
NAFDAC dismantled a major network of covert warehouses in Nigeria that stockpiled over 10 million doses of counterfeit and banned medicines, including critical emergency drugs like injectable anti-malarials.