Search:
Filter:
Sort:
A patient at a North Dakota med spa told investigators she had to coach the clinic owner through the IV administration process. The owner allegedly presented herself as a registered nurse, despite holding no medical licenses.
Prescription Drug Affordability Board Activity, December 2025 Activities Summary Colorado: In October, Colorado’s PDAB heard testimony about its decision to set an upper payment limit on Enbrel, and set price of $600 per 50mg/1ml, effective January 1, 2027. The board’s next meeting is January 9, 2026. Maryland:The PDAB met on December 8, 2025 to review a draft of its annual…
The bill will protect Iowa’s patients by closing enormous regulatory gaps in the med spa and wellness industry.
The agency has found websites and social media advertisements displaying official Health Canada logos with fake endorsements to mislead consumers, and said that it doesn’t endorse health products or permit its logo to be used in ads that promote them.
Reporting on PSM-led research led Google to suppress pill press ads, Congressman Raja Krishnamoorthi sent letters to shippers of weight loss drugs and API, and a former nurse was charged with distributing counterfeit Ozempic.
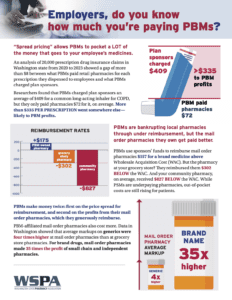
Employers, do you know how much you’re paying PBMs? Pharmacy benefit managers (PBM) tactics have increased costs for patients and healthcare purchasers all while driving community pharmacies out of business and failing to deliver on their promise to lower medication costs. Learn more about these tactics and what states are doing to address them.
Why are price caps a bad solution for medicine affordability? Americans need affordable access to medicines, but upper payment limits (UPLs) set by prescription drug affordability boards and Medicare Maximum Fair Prices (MFPs) are not viable solutions. In our complex drug supply chain, these price caps could bankrupt pharmacies and reduce patient access. Read our handout for other strategies, and…
As of the first week of January, we found several companies with multiple ads still running well past its self-imposed deadline. Google can do better, but will they?
The FDA has posted notices for two nationwide dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
New York found widespread safety violations in med spas, and FDA inspectors cited a Texas distributor for violating the DSCSA.