November 24, 2025: Surveys examine online pharmacy use and patients’ perceived safety.
Major stories
Surveys document consumer behaviors when purchasing medication from online pharmacies, finding that the public may not be aware of the dangers of IOPs.
The Alliance for Safe Online Pharmacies (ASOP) conducted a survey to assess how Americans perceive, purchase, and evaluate the safety of prescription medicines sold online. They found 38% of U.S. adults have purchased prescription medicines online, and that 47% of those who have purchased medicines online have taken that medicine without being fully confident it was as safe as the medicine they would find at their local pharmacy. They also found that 59% of online purchasers report buying medicines they believed were shipped from or intended for sale outside the U.S., and that 65% of U.S. adults falsely believe all websites offering online Rx/health services are reviewed/approved by FDA or state regulators.
In another survey published in the Scandinavian Journal of Primary Health Care, researchers found that over half of the respondents reported using an online pharmacy, but under 10% of participants knew how to identify the logos denoting a safe online pharmacy. They found that women and highly educated people were more likely to use an online pharmacy, and that the majority stated that external factors did not influence buying patterns.
Domestic News
Senator Cotton urges the DOJ to investigate counterfeit drugs. Anchorage doctor accused of health care fraud pleads guilty. Study about patient experiences with AFPs. CSIS explains how counterfeit drugs threaten U.S. health and innovation.
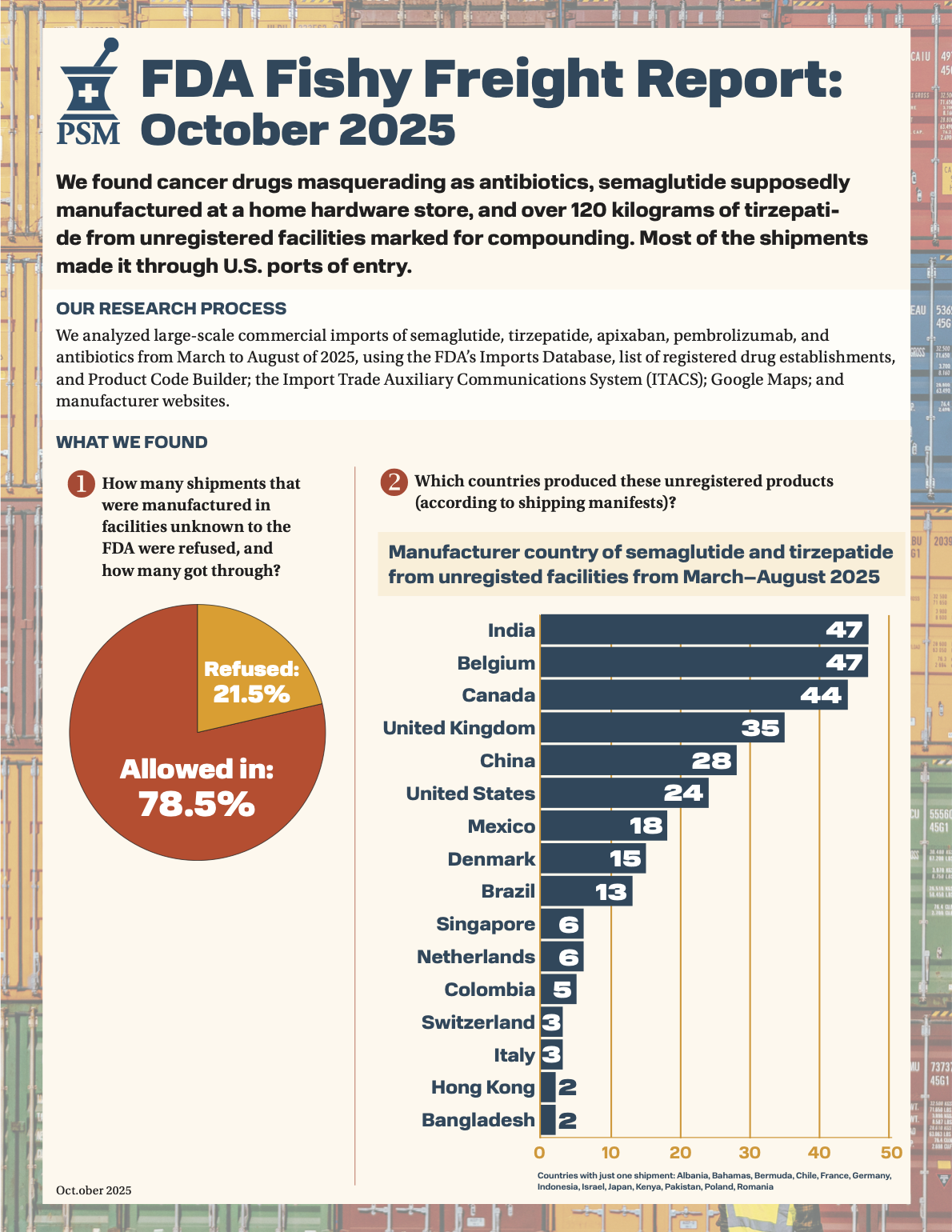
Senator Tom Cotton sent a letter to U.S. Attorney General Pam Bondi, urging the DOJ to investigate the surge in counterfeit drug ingredients entering the U.S., citing PSM's Fishy Freight report, which found shipments of GLP-1 API from unregistered facilities entering the U.S. In the letter, Cotton asks AG Bondi to follow up about the actions the agency has taken regarding counterfeit drugs, what it is doing to disrupt criminal organizations, and what criminal activities are being funded through the counterfeit drug trade.
Want to know more about the suspicious shipments we found? Visit our pharmaceutical border security page to find all our reports from February to October.
On November 18, 2025, an Anchorage doctor and her husband pleaded guilty to health care fraud and attempting to evade and defeat taxes. According to court documents, Dr. Claribel Tan and her husband, Daniel Tan, who operated a rheumatology clinic, allegedly underdosed patients, injecting them with free samples or a different medication than prescribed, injecting them with expired medication, and/or injecting them with medications purchased by other patients. The Tans then billed insurance plans as if Dr. Tan had provided each patient a proper injection.
In 2024 and 2025, the U.S. Attorney’s Office seized approximately $10,471,106 in health care fraud proceeds from the Tans. As part of their plea agreements, the Tans agreed to forfeit those funds to the U.S. and agreed to pay an additional $6,300,849 towards their expected restitution judgment. The Tans further agreed to pay the U.S. an additional $1,855,144 to settle civil claims under the False Claims Act arising from the health care fraud scheme.
A study published in the Journal of Managed Care & Specialty Pharmacy examines patient experiences and access to specialty medicines with alternative funding programs. Just over half the patients reported being uncomfortable with the benefits manager from the AFP vendor. Many reported experiencing treatment delays, which may have been linked to disease progression. Read our resource about AFPs here.
The Center for Security and International Studies (CSIS) released an explainer about how counterfeit drugs threaten U.S. health and innovation, citing PSM’s fishy freight report. In the explainer, they talk about the health impacts of counterfeit drugs, how online pharmacies widen their impact, and how pharmaceutical companies see diverted revenue and weakened IP protections from illicit products, as well as how policymakers can address the problem.
Med spas
On November 14, 2025, the Ohio Board of Pharmacy suspended Luxe Laser’s license to be a terminal drug distributor, citing possession of illegal drugs, including GLP-1s. They also found compounded syringes of GLP-1s and vitamin B-12, despite staff not having the training to do so. Inspectors also said Botox was stored in a freezer, well below the recommended temperature to store the drug at, and a “significant quantity of expired drugs was found throughout the active drug stock storage areas.” Read more about med spas on our page dedicated to the clinics.
Pill presses
A Rhode Island man was sentenced to 120 months in prison and four years of supervised release after a 2022 investigation led to the seizure of multiple kilograms of a mixture and substance containing methamphetamine, 25,000 pills, and five pill presses.
Authorities arrested a man in Massachusetts after recovering a pill press and large quantities of counterfeit pills tied to a Lawrence-based drug operation. Investigators say the equipment and materials were used to manufacture fake Percocet pills containing fentanyl for distribution. While on pretrial release, he allegedly sold over 1,500 grams of counterfeit pills to an informant.
Police and DEA agents seized a pill press used to make counterfeit medication during coordinated raids on two Enfield, Connecticut, homes. The operation uncovered large quantities of drugs and firearms, leading to the arrests of three suspects.
Regulators protecting patients in the news
The FDA issued four new warning letters to companies in New Jersey, Wisconsin, Florida, and Washington for failing to test products for diethylene glycol or ethylene glycol. This comes as counterfeit cough syrup, likely contaminated with the toxic substance, has killed 24 in India.
The FDA also issued warning letters for unapproved analgesics, undeclared NSAIDs, unapproved drugs, and CGMP violations, including violations from an API manufacturer.
Patient safety issues in the GLP-1 space this week

A community alert in a Telegram chat warns that certain retatrutide kits may contain an unknown substance after testing showed zero active ingredient. The message stresses that the kits may contain another unknown and potentially dangerous substance, which is likely given the lack of an active ingredient, and urges users to stop using them immediately and to seek medical attention if they feel unwell after a recent injection, as the adverse reactions have been rapid and severe.
Legislative updates
In New Jersey, state legislators have reintroduced a bill to establish a wholesale prescription drug importation program that is open to Canada and any other foreign country that “uses similarly high standards to regulate prescription drugs.” Drug importation programs undermine the U.S. track-and-trace system, increasing the risk that counterfeit or unsafe medicines enter the legitimate drug supply, and demand may not be able to be supported. Florida is the only state to pass similar legislation, and the program has cost the state $132 million while having never imported a single pill.
New Hampshire has introduced legislation aiming to regulate PBMs by increasing transparency and accountability by banning spread pricing, adopting pass-through pricing, and ensuring all manufacturer rebates go toward lowering premiums or point-of-sale costs.
The Fraternal Order of Police wrote to Representatives Vindman and Crenshaw, urging them to support H.R. 5744, the “Targeting Online Sales of Fentanyl Act.” This legislation would direct the Government Accountability Office to investigate the methods used to enable online sales of fentanyl and assess the procedures and efforts of Federal law enforcement and online providers in combating these online sales.
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
International News
Fake Viagra in Toronto. Counterfeit sedatives are suspected of having killed 27 in the Netherlands. Fake asthma inhalers in India.
Health Canada seized counterfeit Viagra being sold at a Toronto convenience store after the manufacturer confirmed the tablets were fake. Officials warned that the unregulated product could contain incorrect dosages or dangerous contaminants, posing serious health risks.
At least 27 people in the Netherlands are dead, and counterfeit sedatives and sleeping pills purchased from an illegal online store are likely the culprit. The store was run by two Limburg residents who lacked authorization to sell such drugs, which were sold to several teenagers.
In India, multiple drug licenses have been suspended over stocking counterfeit medications, including asthma inhalers.