Prescription Drug Freight Fraud Report, September 2025

Screenshot of a listing for bulk tirzepatide sales, September 2025
Our analysis of data from June and July revealed hundreds of pharmaceutical shipments that entered the U.S. from facilities no one would expect, including unregistered Chinese exporters and alternative medicine clinics abroad. We found 122 shipments of semaglutide, tirzepatide, and pembrolizumab (the active ingredient in the cancer drug Keytruda), and 280 shipments of antibiotics from facilities that do not appear in the FDA’s Drug Establishments Current Registration Database. Some of these were more concerning than others, including large shipments of tirzepatide from a company that has since been added to the FDA import alert list, and chemotherapy and psychiatric drugs mislabeled as antibiotics and granted entry into the United States.
Which American businesses are purchasing mass quantities of tirzepatide from unregulated sources?
Among 258 total tirzepatide shipments in June and July, we identified 25 with manifests declaring that they had been made in facilities that were not registered with the FDA. 20 of those made it into the United States. It is easy to focus on the smugglers, but who ordered these? It’s much easier to prosecute purchasers on U.S. soil, with U.S. bank accounts, and U.S.-based staff, than foreign nationals operating in countries without extradition treaties.
We discuss the largest of these shipments, 10 kg of tirzepatide labeled “Active Pharm Ingred/Chems for Rx Compounding,” below. Ten kilograms is enough tirzepatide for four million starting doses; it clearly went to someone intending to compound it and dispense it to patients. The most likely recipients of these disallowed compounding ingredients are unlicensed compounders, but that’s not the only possibility.
While we’d like to believe licensed compounders would never do this, transparency in licensed GLP-1 compounding API is not great for patients or patient safety advocates. Compounders have been credibly accused of compounding with unapproved ingredients, such as:
- Compounders using semaglutide salts to make semaglutide (FDA originally raised this issue in June 2023)
- FDA inspection of a major national compounder found to be using unapproved API (Empower Pharmacy inspection, October - December 2023)
Regardless of who is ordering these unsafe ingredients for compounding, we agree with 38 state attorneys general that this is a substantial problem that requires a response from state law enforcement and the FDA.
Read the letter 38 attorneys general sent to the FDA in February.
Our research method
We began our research by gathering June and July 2025 entries for semaglutide, tirzepatide, pembrolizumab, and antibiotics in the FDA’s Imports Entry Data Search (1). Then, we cross-referenced facilities associated with imports of semaglutide, tirzepatide, pembrolizumab, and antibiotics with the FDA’s Registered Drug Establishments list using the manufacturer FEI number, the manufacturer name, or the manufacturer address.
Through this process, we discovered 11 shipments of pembrolizumab (commonly known as Keytruda), 86 shipments of semaglutide (commonly known as Ozempic, Wegovy, and Rybelsius), 25 shipments of tirzepatide (commonly known as Zepbound and Mounjaro), and 280 shipments of antibiotics made in facilities that were not registered with the FDA.
Why does this matter? A facility not registered with the FDA will never be inspected by the FDA. For almost all medicines, that makes it illegal to ship into the U.S. and unsafe to give to patients.
For every semaglutide, tirzepatide, and pembrolizumab shipment from unregistered facilities, we looked up the shipment ID in the Import Trade Auxiliary Communications System (ITACS) to examine a more detailed manifest of what else was included in each container, validate declared quantities, and uncover inconsistencies. This helped us better understand the context of the semaglutide, tirzepatide, and pembrolizumab shipments. You can learn more about ITACS in this FDA industry presentation. We also found more information about the intended use of the drugs using the FDA’s Product Code Builder.
In the antibiotics category, we looked for classification mismatches between FDA imports product code descriptions and the in-depth manifests in ITACS. There were 45 cases where compounds labeled as “antibiotics” were not antibiotics at all.
(1) We did not do a comprehensive search of every single import in the database, just four drug categories we have seen a lot of fraudulent activity around.
What we found
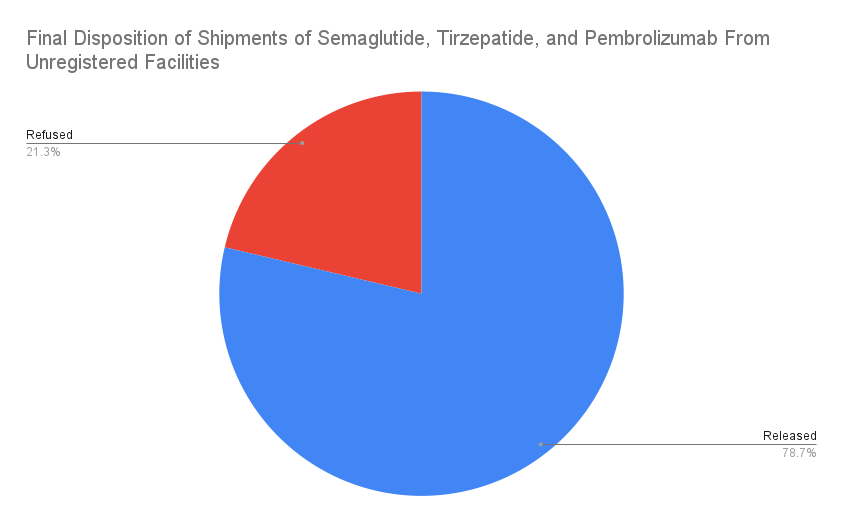
Our analysis of semaglutide, tirzepatide, and pembrolizumab included 575 shipments in total. Of these, 122 shipments were linked to unregistered facilities: 86 of semaglutide, 25 of tirzepatide, and 11 of pembrolizumab. Nearly 80 percent of these shipments were released into the U.S. supply chain despite their questionable origins. Several of these shipments stood out as particularly alarming.
Could the codes be wrong? Is there an incentive to lie? If a shipper lies on a manifest, it doesn’t matter what the contents are, it is grounds for seizure or refusal of the shipment. As a result, shippers and foreign manufacturers are incentivized to be honest in these documents unless they are doing something so egregious that they want to risk refusal or seizure.
An FDA import alert to protect Americans
At the end of May, a non-FDA unregistered company in China, Chengdu Miao Ding Xin Pharmaceutical Technology Co., Ltd., shipped nearly 10 kilograms of tirzepatide—four million starting doses—in three separate shipments intended for compounding (Shipment IDs: H77-0230371-7/13/1, H77-0230371-7/13/2, H77-0230371-7/13/3). These shipments were marked with product code 61PCA72, “Active Pharm Ingred/Chems for Rx Compounding.” This was not an accident; the manufacturer and shipper chose this coding from dozens of other options to identify an intended usage on their manifest.
Those shipments were released and made it into the country, but the FDA added Chengdu Miao Ding Xin Pharmaceutical Technology to the FDA import alert list on July 3, 2025. This is a success: agency staff will be able to rapidly detain future shipments of this company’s products without having to test or physically examine them.
A homeopathic clinic
In another case, 10 pieces of Rybelsus, the brand-name oral version of semaglutide, were shipped from Dr. Nanduri’s Homeopathic Clinic in India.
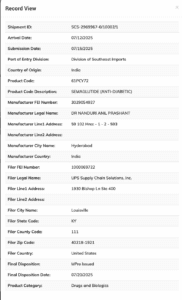
The import record for shipment SCS-2969967-0/10002/1
9 KILOGRAMS of tirzepatide
There was also a huge nine-kilogram shipment of tirzepatide from a manufacturer we flagged in our last Freight Fraud report, Hangzhou Thinheal Pharma-Tech Co.
Customs and Border Protection stopped a 10-kilogram shipment listed for further compounding in May, but they didn’t catch this shipment in July.
The July shipment may have slipped through because it indicated that it was intended for “sampling and testing” in the ITACS declaration. Nine kilograms seems like an awfully large shipment for that purpose. While we can’t be certain, we suspect that the shipper used this code in an effort to evade inspection.
May Shipment ID: BYA-2501606-4/12/2:
July Shipment ID: 879-4076250-0/10003/1:
Package drop off
A smaller shipment from Mexico used what appears to be a shipping drop-off location as its manufacturing address, raising serious questions about the legitimacy of its origin.
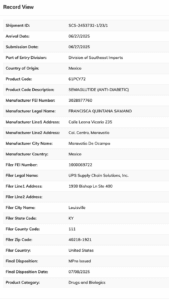
The import record for shipment SCS-2453732-1/23/1

Google maps
Frame store
This shipment declared 45 pieces of semaglutide from Romania, with the listed manufacturer’s address corresponding not to a pharmaceutical facility but to a frame shop. (See the FDA import record, SCS-1582320-1/122/1)
Antibiotics that are…not antibiotics and not made in FDA-registered facilities
The antibiotics category showed a different but equally troubling pattern. We reviewed 515 shipments labeled as antibiotics and found that 280 came from unregistered facilities. More than 45 shipments were not antibiotics at all, yet more than 90 percent of those misclassified products were released into the U.S. Some examples of these mislabeled imports included:
- 5-azacytidine, a chemotherapy drug used to treat leukemia since the 1970s (Shipment IDs: 799-7374291-0/12/1, 799-9734889-2/13/1, 799-9753233-9/13/1, 799-0004861-4/10003/1)
- Rapamycin, an immunosuppressant commonly prescribed for organ transplant patients (Shipment ID: 336-5726740-3/62/1)
- Fenofibrate, a cholesterol medication (Shipment ID: 799-9084120-8/33/1)
- Zonalta, a psychiatric medicine for bipolar disorder. (Shipment ID: SCS-1314515-1/32/1)
In addition, dozens of shipments contained antifungals, antidiarrheals, and even homeopathic concoctions. None of these are antibiotics, but only three of the 45 shipments were stopped.
We theorize that smugglers code their shipments as antibiotics because they have discovered that shipments labeled as antibiotics receive less scrutiny. This is a risky tactic because lying on a shipping manifest in an easy-to-spot way leads to an automatic refusal or destruction.
Where can I learn more?
Review our past Freight Fraud reports here.
You can also look up shipments yourself using the shipment numbers provided in ITACS and in the FDA’s shipment database. For more detail and to see the shipments we flagged in this report, see the source documents.