News Coverage
The Partnership for Safe Medicines has been publishing information about the counterfeit drug problem around the world for more than a decade. With experts leading the organization and a committed and passionate set of writers and editors, our content is more in-depth than many other sources, which simply copy links to the news from other websites.
Washington State investigators found serious violations at a Mochi Health-affiliated compounding pharmacy producing GLP-1s, and a med spa bill in Indiana hears public testimony about a med spa bill.
Industry experts told us that these two companies are on the FDA’s Green List, despite problematic inspections in late 2024 and early 2025. Learn what the inspectors found.
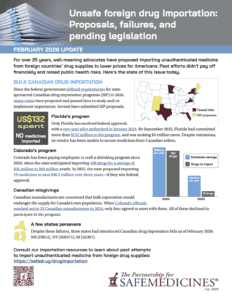
Well-meaning advocates continue to propose importing medicine from foreign countries to lower drug prices for Americans. Past efforts didn’t pay off financially and raised public health risks. Here’s the state of this issue today.
A Pennsylvania compounding pharmacy received another FDA warning letter following a series of enforcement actions, including a $1 million state fine in October.
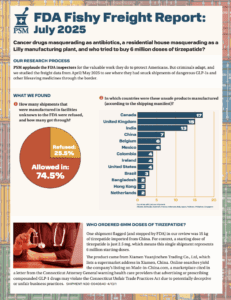
The last two months of 2025 saw thousands of shipments of drugs popularized by social media imported into the U.S., as well as Xarelto and antibiotics. The shipments carried a wide range of product codes; some appropriate, and others far less so.
On February 17, PSM submitted written and oral testimony in opposition to Colorado HB26-1056, which would permit self-insured employers to use alternative funding programs to import foreign medicines from outside the U.S. drug supply chain.
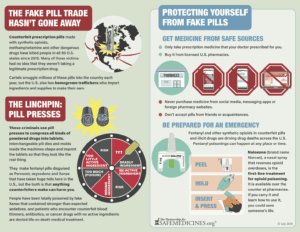
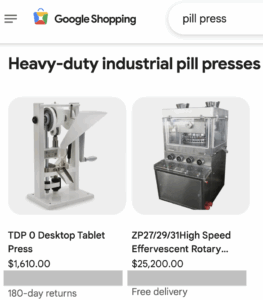
Unregulated pill presses and molds make fake pills with dangerous and even deadly consequences for thousands of people every year. Read our July 2025 to January 2026 update about pill press seizures, policy developments, and legislation.
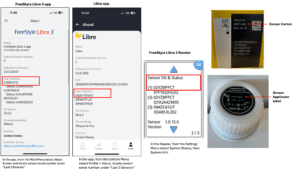
The FDA has posted a notice about the recall of continuous glucose monitors from Abbott.
The federal spending package that ended January’s government shutdown will prohibit PBMs from linking rebates to drug companies’ list prices. The FTC reported that PBMs inflated the prices of generic drugs.
The FDA has posted a notice for a dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
PSM Executive Director Shabbir Imber Safdar released the following statement in response to news that Hims & Hers is now compounding pill versions of a newly approved GLP-1 weight-loss drug.
This post outlines practical steps Congress should take to strengthen pharmaceutical border security, reinforce regulatory oversight, and protect patients.
A patient at a North Dakota med spa told investigators she had to coach the clinic owner through the IV administration process. The owner allegedly presented herself as a registered nurse, despite holding no medical licenses.
The bill will protect Iowa’s patients by closing enormous regulatory gaps in the med spa and wellness industry.
The agency has found websites and social media advertisements displaying official Health Canada logos with fake endorsements to mislead consumers, and said that it doesn’t endorse health products or permit its logo to be used in ads that promote them.
Reporting on PSM-led research led Google to suppress pill press ads, Congressman Raja Krishnamoorthi sent letters to shippers of weight loss drugs and API, and a former nurse was charged with distributing counterfeit Ozempic.
As of the first week of January, we found several companies with multiple ads still running well past its self-imposed deadline. Google can do better, but will they?
The FDA has posted notices for two nationwide dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
New York found widespread safety violations in med spas, and FDA inspectors cited a Texas distributor for violating the DSCSA.
Our analysis of data in September and October 2025 revealed that large-scale freight fraud involving GLP-1s, oncology drugs, and other high-demand pharmaceuticals continues unabated.
CBP reported seizing 450 shipments of illegally imported contact lenses and prescription medicines. Dozens of state AGs asked Meta to enforce policies against misleading weight loss products.
The med spa owner admitted to using unapproved injectables that sickened at least 10 patients. New York authorities announced the results of a 2024 crackdown on illegal med spas. PSM published in The Journal of Illicit Trade, Financial Crime, and Compliance.
PSM wrote the House Energy & Commerce Committee in support of the Safeguarding Americans from Fraudulent and Experimental (SAFE) Drugs Act, to help protect the American drug supply from unsafely compounded medication.
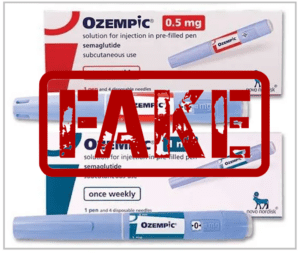
FDA announced the seizure of dozens of counterfeit Ozempic 1mg injection pens labeled with an authentic lot number, PAR1229, but displaying the expiration and lot numbers for the product in the wrong place.
The FDA and Novo Nordisk are warning the public about counterfeit Ozempic injections circulating in the legitimate U.S. drug supply chain. The FDA announced the seizure of multiple batches of counterfeit Ozempic 1 mg injections, labeled with lot number PAR1229, on December 5, 2025. Their contents and safety are unverified and pose serious health risks.
MMWR field notes about Americans hospitalized after self-injecting imported Botox raise serious concerns about patient safety.
Surveys document consumer behaviors when purchasing medication from online pharmacies, finding that the public may not be aware of the dangers of IOPs.
What are the most important lessons from CNBC’s documentary about alternative funding programs (AFPs)?
CNBC aired a documentary about alternative funding programs that included interviews with PSM’s Shabbir Imber Safdar, HSI and FDA officials, as well as a representative from Gilead Sciences and from the companies supplying these drug.
The FDA warned websites in five countries to stop selling Americans unapproved cosmetic injections.
PSM sent a letter explaining how H.R. 5316 undermines critical patient safety protections.
The Maryland distributors were convicted at trial in Florida. A federal judged sentenced a pill press seller from China.
Authorities stated this was the largest single seizure of trafficked weight loss medicines worldwide.
Board member Dr. Kenneth McCall was named an associate editor of the Journal of Pharmacy and Pharmaceutical Sciences; Executive Director Shabbir Safdar joined the editorial board of the Journal of Illicit Trade, Financial Crime, and Compliance.
A study says IV hydration spas need more oversight. Louisiana residents were hospitalized after receiving black market botulinum toxin injections. Hulu delved into the counterfeit GLP-1 market and Fierce Healthcare covered AFPs.
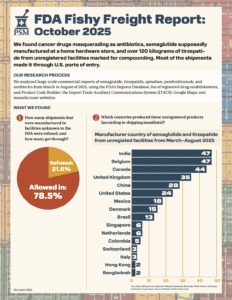
What large-scale commercial imports made it into the country between March and August of 2025?
This summer, Brian Mayo, Executive Director of the Oregon State Pharmacy Association (OSPA), made the case for pharmacy benefit manager (PBM) reform as a better way to rein in drug prices.
PSM Executive Director Shabbir Safdar is speaking at the Democratic Governors Association about the threat of unregulated prescription drugs smuggled into the U.S.
Supply chain complexity means that upper payment limits and Medicare maximum fair prices will fall disproportionately on pharmacies, leading to closures that threaten patient access to care.
Although the state won FDA approval for its importation program in January 2024, it has yet to implement the program.
Medical spas and wellness clinics are operating on the edge of existing regulatory frameworks and it’s a risk to American patients.
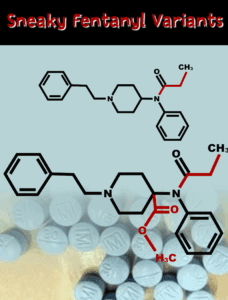
Between November 2022 and June 2023, Amarvel Biotech sold over 200 kilograms of precursor chemicals to U.S. buyers with full knowledge that they would be used to make fentanyl.
Our analysis of data from June and July revealed hundreds of pharmaceutical shipments that entered the U.S. from facilities no one would expect, including unregistered Chinese exporters and alternative medicine clinics abroad.
The warning letter was one of about 100 is a new focus on deceptive direct-to-consumer drug advertisements.
The “green list” will help protect patients from unreliable, illegally compounded GLP-1s and simplify screening for border security.
PSM led a coalition of organizations urging leaders of the Senate HELP and House Energy and Commerce committees to help strengthen the FDA’s ability to protect Americans from unsafe and counterfeit medicines and medical products.
A decade ago no one had heard of deadly counterfeit prescription pills. Victims’ families bravely spoke up, lobbied, and rallied for change.
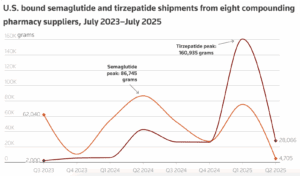
Chinese companies that supplied U.S. compounders have turned to making generic semaglutide for markets where Novo Nordisk’s main patent is expiring in 2026.
Elle published an exposé about bad actors and loose regulation in the medical spa industry.
On August 7, the Partnership for Safe Medicines and ADAP Advocacy Association filed a complaint asking the New York State Board of Pharmacy to take regulatory action against a pharmacy that allegedly sold counterfeit HIV medicine to a Queens, New York patient.
PSM and ADAP Advocacy Association filed an official complaint with the State of New York regarding allegations that City Plus Care Pharmacy Inc. dispensed counterfeit HIV medication to a patient in Queens, New York.
Statistics in the agency’s Report on the State of Pharmaceutical Quality show that active pharmaceutical ingredient (API) sites that solely supply compounding pharmacies are disproportionately high risk.
The proposed updates aim to help address safety concerns created by pharmacies reselling compounded sterile drugs purchased from outsourcing facilities.
The Pennsylvania Attorney General filed charges against a pharmacy owner and the Ohio Board of Pharmacy revoked licenses to protect patients.
The newest Fishy Freight report continued to find suspect API shipped into the U.S. while a report from the United Nations’ Office of Drug Crime highlighted the dangers of contaminated and counterfeit medicines made with diethylene glycol and ethylene glycol.
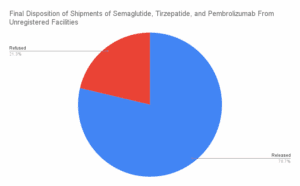
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
We’ve published the final document of best practices for online pharmacy-to-pharmacy marketplaces.
The Partnership for Safe Medicines (PSM) today announced its strong support for the reintroduction of the Cooper Davis Devin Norring Act, a bipartisan effort aimed at curbing the online sale of deadly counterfeit and illicit drugs.
A policy change announced by U.S. Customs and Border Protection means that all FDA-regulated products are now subject to inspection at ports of entry.
503B outsourcing facilities make our drug supply more resilient, but 81% of 503Bs newly registered since June 2021 have never been inspected by FDA staff. Learn why we recommend changes to this important program.
Pill presses and molds are used to make fake pills with deadly consequences thousands of people every year. The number of Americans that take fake pills annually without a fatal event is even higher. Read our January through June 2025 report covering pill press seizures, policy developments, and legislation.
Avanish Kumar Jha and Rajnish Kumar Jha got 30-month sentences for selling counterfeit medicines—including vials of fake Keytruda—to U.S. buyers.
After a November 2024 inspection, the FDA issued a Form 483 to Empower Clinic Services highlighting violations related to compounded drug manufacturing practices at its East Windsor, New Jersey facility.
The change will affect the Google Ads and Google Shopping platforms starting in September 2025.
A July 1, 2025 post clarifies that applicants can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
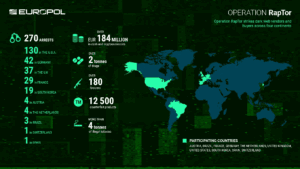
The operation launched 1,728 investigations in 91 countries and shut down 13,000 criminal-linked websites, social media pages, channels, and bots used to market and sell illegal or counterfeit medicines.
On June 26, PSM commented in support proposed revisions to forms FDA 3500 and FDA 3500B, which are used to report adverse events from prescription and over-the-counter medicines.
More than 40 cases of botulism linked to Botox injections have been reported in the U.S. and U.K. over the last two months.
The bill permanently schedules fentanyl analogues as Schedule I drugs under the Controlled Substances Act.
The Jha brothers admitted selling counterfeit Keytruda and other prescription drugs to undercover agents.
PSM is soliciting feedback on draft best practices until June 20th. Please review the guidelines and send your thoughts to editors@safemedicines.org.
The FDA warned companies over the sale of an unapproved medical device and contaminated eye medicines, and disbarred a Texan who sold illegally imported pharmaceuticals.
The FDA has announced a voluntary nationwide recall of two dietary supplements sold exclusively online by www.umary-usa.com. Lab testing revealed that Unavy Ácido HIALURÓNICO and Umovy Ácido HIALURÓNICO contain undeclared active pharmaceutical ingredients: diclofenac, dexamethasone, and omeprazole.
Federally-registered compounding facilities stopped making tirzepatide on May 22. This transition is significant for patients, and we at PSM think there are four things you should be watching for.
Operation RapTor led to 270 arrests and the seizure of more than $200 million in currency and digital assets.
Articles in Endpoints News and the Houston Chronicle raise questions about the regulatory process for 503B compounding facilities.
Exposes in the Houston Chronicle and Endpoints News examined Empower Pharmacy’s safety record. A grand jury indicted a Chinese company and three of its employees for pill press and counterfeit die mold sales.
The FDA wants to ensure that domestic and foreign companies receive the same level of regulatory oversight.
Catch up on research PSM released about medicines imported from unregistered sources.
What if your blood thinners were made in an unverified location in Colombia? In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect.
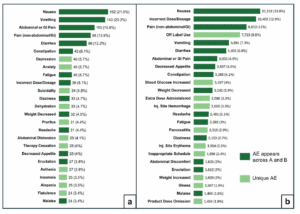
New research shows a correlation between compounded weight loss and diabetes injectables and increased adverse events. GIlead Sciences is suing another New York City pharmacy that allegedly sold counterfeit HIV medicine.
The FDA has issued a warning to the operators of the online pharmacy www.thesafepills.org for illegally selling unapproved and misbranded opioid medications, including tapentadol products marketed as Aspadol and Typendol. These unauthorized drugs, sold without a prescription, pose significant health risks such as overdose, addiction, and even death.
One of SafeChain’s three owners pleaded guilty. The company collected secondhand HIV medicines from patients and resold them to licensed U.S. pharmacies, forging paperwork to make them look legitimate.
A Kentucky doctor treated weight loss patients with research chemicals; CBP seized 90,000 alprazolam pills being smuggled into the U.S.
The FDA and Novo Nordisk are warning the public about counterfeit Ozempic injections circulating in the U.S. drug supply chain. The falsified products, labeled with lot number PAR0362 and serial numbers beginning with 51746517, were seized by the FDA on April 9, 2025. Their contents and safety are unverified and pose serious health risks.
PSM is seeking input on developing a set of best practices to reduce sales of counterfeit and diverted medicines on online pharmacy-to-pharmacy marketplaces.
The FDA announced that it had seized counterfeit Ozempic injections on April 9. PSM testified at a congressional hearing.
A study examined the 130 enforcement actions undertaken by the U.S. Food and Drug Administration’s Office of Criminal Investigations from 2016 through 2021.
The state of Colorado submitted a revised application to the U.S. Food and Drug Administration to operation a Canadian drug importation plan in March. This blog post examines how the state’s application has changed over the years.
A March 12, 2025 memo said the state had paid more than $150,000 defending itself in a lawsuit Amgen had filed over plans to set an upper payment limit on its rheumatoid arthritis treatment, Enbrel.
Senator Jim Banks asked pointed questions about how FDA will stem the tide of semaglutide and tirzepatide coming from unknown facilities.
“Alternative funding programs” are shifting expensive prescription coverage to illegal drug importation schemes.
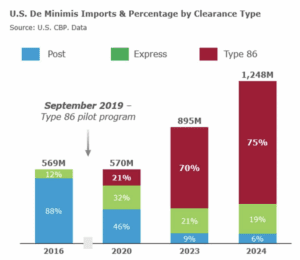
On March 14, the Partnership for Safe Medicines submitted comment on Customs and Border Protection’s proposed update to regulations around low-value, “de minimus” shipments.
A Texas man billed government programs millions for creams compounded by untrained teenagers. News about counterfeit medicine in six U.S. states.
FDA’s shutdown of GLP-1 compounding could lead to a new era of risk for U.S. patients.
The FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
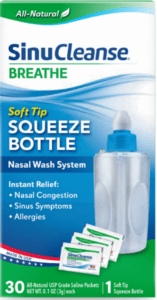
An FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
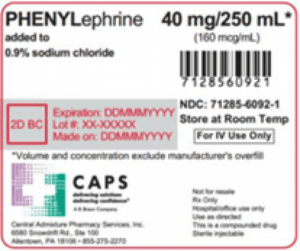
An FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
An FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
The U.S. Attorney’s Office in the Western District of Washington has charged two Indian nationals with selling Americans counterfeit Keytruda.
There has been controversy about the ad Hims & Hers ran during the Super Bowl. And now an SEC filing says investors should be warned about risk if their ads are found to be non-compliant.