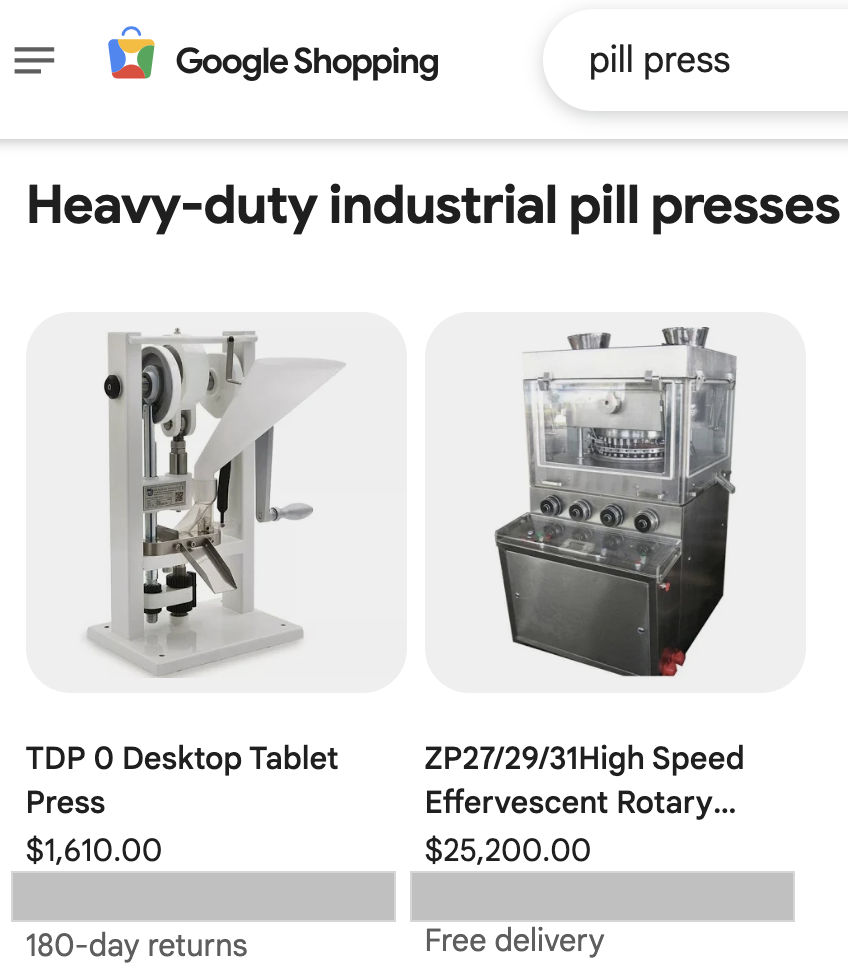
July 7, 2025: Google to block promotion of pill presses on ads and shopping platforms
Major Stories
On July 1, Google announced that it was updating its dangerous products and services policy to restrict the promotion of pill presses, encapsulating machines and components they use, such as dies, molds and stamps used to create or imprint pills. The change will affect the Google Ads and Google Shopping platforms starting in September 2025, with six weeks until full implementation.
The U.S. Food and Drug Administration (FDA) announced that states and tribes proposing importation programs can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
Domestic News
FDA warned a compounding facility over poor conditions and unregistered suppliers. A judge ruled that CVS Caremark inflated Medicare drug prices.
Read FDA's warning letter to Staska Pharmaceuticals.
A federal judge in Pennsylvania ordered CVS Caremark to pay $95 million for inflating Medicare drug prices, after the court found that the pharmacy benefit manager paid pharmacies negotiated prices, but reported higher individual transaction prices to insurers, who passed those numbers to the Centers for Medicare & Medicaid Services. Federal law requires that Medicare subsidies be based on the prices actually paid to pharmacies.
An American Medical Association report on prescription drug affordability boards (PDABs) took no position on the effect of upper payment limits on physician reimbursements and patient access to medicine, stating that there was no research available to evaluate impacts or outcomes of such policies.
The FDA posted the warning letter it sent on May 5 to a Nebraska-based compounding facility where agency inspectors found unsanitary conditions and API from an unregistered supplier. The facility recalled a lot of ascorbic acid solution for injection for the presence of glass particles in September 2024. FDA also posted a warning letter to a website selling unapproved medicines, including opioids and semaglutide.
The Texas Medical board issued a cease and desist order to a woman accused of giving illegal injections to patients at a southwest Houston cosmetic business.
Legislation
The Wisconsin legislature passed SB 45, which would establish an eight-member PDAB with the power to set upper payment limits, as well as a Canadian drug importation program that would import “non-brand-name drugs” with fewer than four competitors in the U.S. market if it would create substantial savings.
Florida’s governor signed S1344, which permits pharmacists or wholesalers working with Dept. of Juvenile Justice to import medicine from an eligible Canadian supplier.
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
Patient safety issues in the GLP-1 space this week
In a post calling for physicians to educate patients about the danger of ordering medicines online, the Institute for Safe Medicine Practices recounted the adverse event a patient experienced after injecting themselves with semaglutide they had purchased online. There was no way to know whether the product was genuine, and it did not have adequate instructions for dosing.
International News
Fake cancer medicines in Africa are raising concerns about drug quality worldwide. Canada warned about unapproved glucose monitors. Counterfeits reported in Lebanon, India and Ukraine.
Health Canada warned people with diabetes and their caregivers that unauthorized blood glucose monitors being sold online may pose serious health risks by giving false blood glucose readings or failing to provide timely alerts.
Researchers testing seven generic cancer medications distributed in Cameroon, Ethiopia, Kenya and Malawi found that 20 percent of their 189 samples were substandard, with most containing too little active ingredient to be effective. The Bureau of Investigative Journalism reported that these brands of drugs have been shipped to more than 100 countries over the last six years.
This news comes as Lebanese courts prosecute a woman who allegedly sold counterfeit cancer medications to patients via 70 pharmacies and a special task force in Uttar Pradesh, India busted a gang smuggling counterfeit Avastin injections.
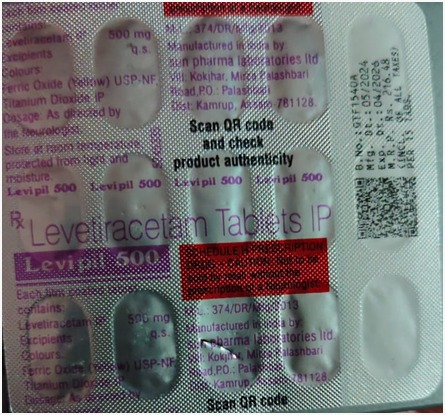
Authorities in Telangana, India seized counterfeit Levipil 500, an anti-epileptic drug, that was falsely labeled as made by Sun Pharma Laboratories.
Law enforcement in Mykolaiv, Ukraine seized medicines from a pharmacy chain suspected of selling counterfeit Ozempic and unapproved eye drops.