Doctors: Protect Your Patients, Your Practice And Yourself
Counterfeit medicine is not just a threat to patients. Doctors who have broken the closed American drug supply chain have served prison sentences, lost their licenses, paid fines and penalties, and been debarred by the U.S. Food and Drug Administration.
Why have Doctors Been Prosecuted?
For Buying Medicines from Unlicensed Sources
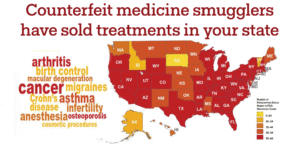 Between 2012 and 2017, the FDA warned more than 3,000 medical practices to stop buying medicines from unlicensed foreign wholesalers who had been caught selling counterfeit drugs. Patients who encounter these drugs go under-treated and untreated, which can be catastrophic; at worst, they may be poisoned.
Between 2012 and 2017, the FDA warned more than 3,000 medical practices to stop buying medicines from unlicensed foreign wholesalers who had been caught selling counterfeit drugs. Patients who encounter these drugs go under-treated and untreated, which can be catastrophic; at worst, they may be poisoned.
Buying non-FDA approved drugs can be catastrophic for medical practices, too. Since 2005 and mid-2018, 59 doctors were prosecuted for crimes related to purchasing non-FDA approved medications, treating patients with illegally imported drugs, and/or fraudulently billing Medicare and private insurance as if they had purchased these drugs legally and at full price. 57 of those prosecuted were fined a combined total of $37.5 million. 16 received prison sentences.
- 2016: Dr. Paul Singh of Tehachapi, California—despite repeated warnings—continued to purchase non-FDA approved intrauterine devices (IUDs) and insert them into his patients without informing them. When patients complained Singh reinserted the same IUD. Singh received a 6-month sentence and a $91,900 fine.
- 2013: Dr. William Ralph Kincaid of Johnson City, Tennessee, received a 24-month prison sentence and was ordered to pay over $2.5 million in fines and penalties for purchasing misbranded oncology medicines. When nurses expressed concerns about treating patients with non-FDA approved drugs, Kincaid sent shipments to a different address to hide his illegal activities.
- 2006: Broward County, Florida doctor Bach McComb received a 36-month prison sentence after he injected three other people and himself with a non-FDA approved version of Botox not meant for use in human beings. His victims described months of being trapped in their bodies on hospital beds while ventilators did the breathing for them.
 For creating their own, non-FDA-approved treatments, often using illegally imported ingredients
For creating their own, non-FDA-approved treatments, often using illegally imported ingredients
2011: San Diego, California physician Kurt Donsbach was sentenced to a year in county jail and 10 years probation for selling fake drugs to patients, practicing medicine without a license and attempted grand theft. Donsbach gave patients—including terminal cancer patients—"natural" supplements made of pharmaceutical ingredients that were not approved by U.S. regulators.
For dealing controlled substances they manufactured themselves

In 2016, Maggie Crowley died after taking one of the pills Dr. Benjamin sold.
2018: After a lengthy investigation, orthopedic surgeon Johnny C. Benjamin, Jr., of Vero Beach, Florida, was sentenced to life in prison after a jury found him guilty on five counts, including participating in a conspiracy to possess with intent to distribute furanyl fentanyl which resulted in death.
Benjamin manufactured and sold counterfeit oxycodone pills using imported furanyl fentanyl and his own pill press.
How Can You Protect Your Practice?
Verify Your Drug Distributors’ Licenses
First and foremost, it is critical to make certain that medical practices are sourcing medicine from state-licensed wholesalers selling FDA-approved medicines.
To discern whether a supplier is legitimate, check with your state licensing board. You can find your state's verification page on the FDA's list of "U.S. State Agencies Responsible for Licensing Wholesale Prescription Drug Distributors."
Most states will let you verify licensed distributors online through an online search. Be sure to check your suppliers’ licenses at least twice a year.
Educate your staff
It's equally important that nurses and office managers understand that ordering drugs from unlicensed suppliers is illegal and dangerous. Your medical practice staff are well-placed to both prevent counterfeit medication use and diagnose the telltale signs of counterfeit drugs in their patients.
Make sure your staff knows how to verify distributor licenses and that they know the warning signs for counterfeit drugs.
Educate Your Patients
Be alert about patients who may be having trouble paying for their medications. Share resources to help them protect themselves from counterfeits, find affordable prescription medications and to save money safely when buying medication online.
More about the threat of non-FDA approved medicine in the U.S.
- Counterfeit Drugs In America: Crimes, Victims & Solutions, our primer about the threat counterfeit medicines pose to Americans
- The Case Against Canada Drugs
- Black Market Cancer Drugs in the U.S., 2007-2018
- Black Market Injectable Cosmetic Treatments, 2005-2013
- Myth: We are getting the same drugs Canadians take. A pamphlet summarizing Maine’s experience with drug importation in 2013-2014.
- Counterfeit HIV Medications in the U.S.

 For creating their own, non-FDA-approved treatments, often using illegally imported ingredients
For creating their own, non-FDA-approved treatments, often using illegally imported ingredients