Between 2007 and 2018, Foreign Wholesalers Sold American Doctors Black Market Cancer Medicine that Was Counterfeit
Doctors thought they were buying safe medicines, but these distributors hoodwinked them. Is that a risk you want to take?
Between 2007 and 2018, American physicians and clinics demonstrated that drug importation is not safe and is extremely difficult to make safe. Doctors thought they were saving money, but they were endangering patients and costing themselves millions in fines. They were purchasing from black market wholesalers posing as licensed distributors in Canada and other countries who sold illegally imported, expired, damaged and outright counterfeit medications—including cancer treatments.
To learn more, read Black Market Cancer Drugs in the U.S., an updated resource which summarizes prosecutions of drug counterfeiters, black market distributors and medical professionals who sold, purchased and even treated patients with non-FDA approved cancer treatments.
Across all therapeutic areas, the FDA has traced criminal wholesaler drug importation businesses to at least 3,000 medical practices across the United States.
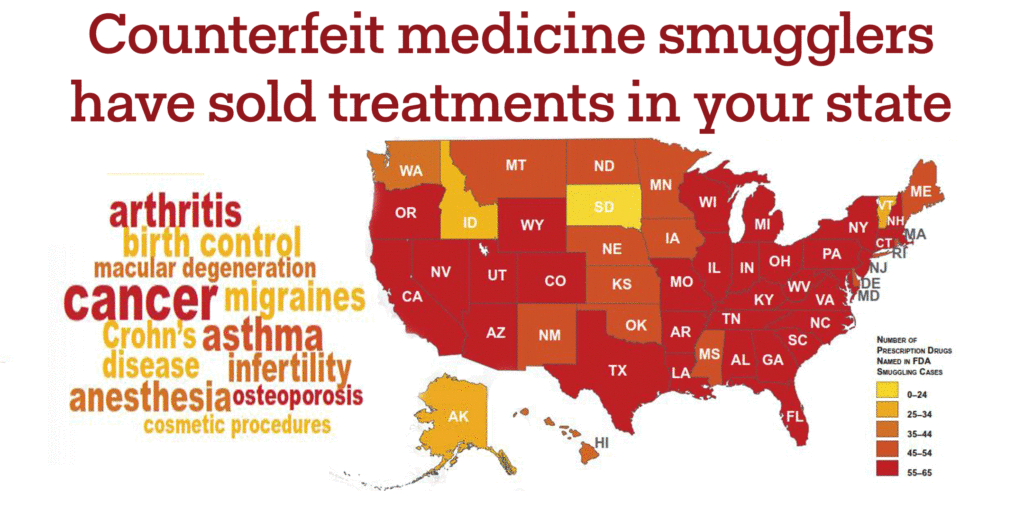
The FDA caught these illegal distributers, but not before their medicines reached patients like Betty Hunter, a cancer patient whose doctor treated her with counterfeit Avastin that had no active ingredient.
Importation is not safe, even at a wholesale level. Only licensed U.S. wholesalers selling FDA-approved medicines are safe for American patients.