July 21, 2025: Inspection policy for all FDA-regulated products changed at ports of entry
Major Stories
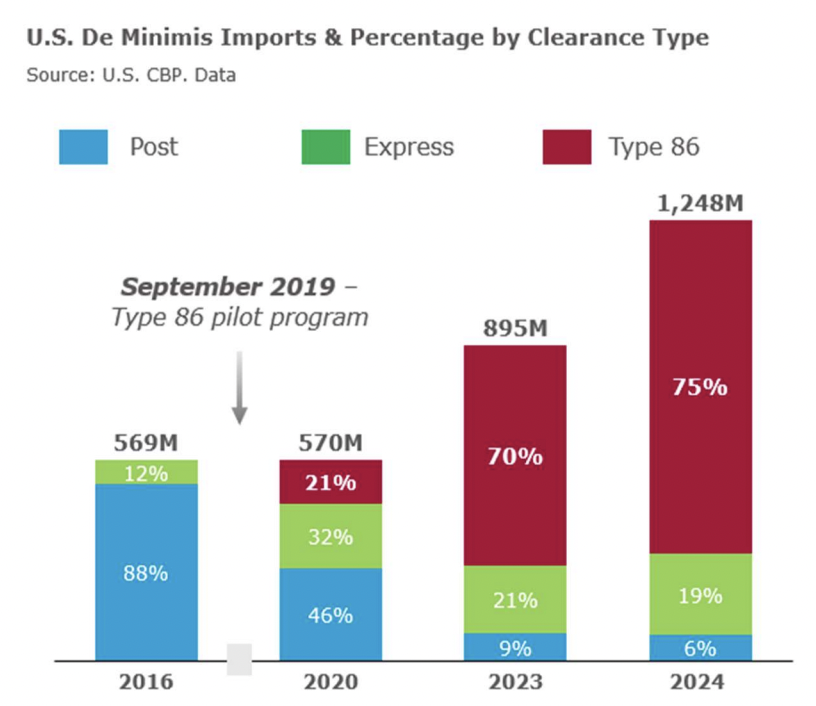
U.S. Customs and Border Protection (CPB) issued an update to the trade community announcing that the U.S. Food and Drug Administration (FDA) will now review all FDA-regulated products at ports of entry, including de minimis shipments (valued at less than $800). CBP cited the advancement in the technological capabilities of both trade partners and the FDA as the reason for this policy change.
PSM submitted a comment in March 2025 for two proposed CBP rules, both of which would address critical gaps in the De Minimus shipment policies. That letter is available here.
Domestic News
HALT Fentanyl Act signed into law, FDA inspects Sun Pharma, and pill press cases in two states.
The HALT Fentanyl Act, federal legislation that permanently categorizes all fentanyl-related analogues as Schedule I in the Controlled Substance Act, has been signed into law. This law gives law enforcement officers greater authority to go after criminals and carries stronger penalties for those convicted of possessing or distributing fentanyl.
The U.S Food and Drug Administration inspected Sun Pharma in June 2025 and cited the company for failing to investigate the source of bacteria found in test vials or deal with damaged equipment that had caused drugs to be contaminated with metal particles.
Police in Port St. Lucie, FL arrested a woman for allegedly operating an unlicensed med spa out of a shed in her backyard. A victim approached police in May, explaining that the Botox injections she received in that shed had left her with facial paralysis. When the victim demanded to see the defendants medical credentials, she was shown an expired phlebotomy license that had been altered to appear it was still valid. Botox is an FDA-regulated product and can only be purchased by licensed medical professionals.
Deiby Felix of Lynn, MA received a 15-year prison sentence after he pleaded guilty to his role in a drug trafficking organization that used multiple industrial pill presses to manufacture counterfeit pills that contained fentanyl and methamphetamine. The other defendants in this case, Emilio Garcia and Sebastien Bejin, received sentences of 18 years and 12 years, respectively, earlier this year.
A federal grand jury indicted a Kansas City, MO man for possession with intent to distribute. While executing a search warrant at the defendant’s home, law enforcement officers seized two pill presses and over six pounds of powdered fentanyl.
Pharmacy Benefit Managers
The Oregon State Pharmacy Association submitted a letter to the Oregon Prescription Drug Affordability Board to rebut the Pharmaceutical Care Management Association's (PCMA) public comments for the July 2025 meeting. Said Executive Director Brian Mayo, "While PCMA attempts to defend the current Pharmacy Benefit Manager (PBM) model, their arguments consistently misrepresent the realities faced by Oregon patients and pharmacies, and fundamentally diverge from the PDAB's core mission to protect Oregonians from exorbitant drug prices."
Mayo cited testimony provided to the U.S. Senate Judiciary Committee that PBM market manipulation has caused more than 40% of prescription drug spending to profit intermediaries, including PBMs, rather than manufacturers, and as a result, PBMs have shareholder returns that are almost twice those of the average S&P 500 company. Mayo gave the example of insulin, a hot topic for the Oregon PDAB, pointing out that "Oregon diabetes patients paid higher out-of-pocket costs while PBMs retained the savings negotiated on their behalf."
Mayo urged the PDAB to eliminate spread pricing and to require 100% rebate pass-throughs. Additionally, he requested the PDAB recommend legislative action on these topics and also prohibit vertical integration and PBM ownership of pharmacies, establish fiduciary responsibility for PBMs to act in clients' best interest, implement price transparency benchmarks, and restrict formulary practices that prioritize PBM profits over patient care.
Legislation
The Obesity Society is hosting a Congressional Briefing July 22, 2025 from 11:30 AM - 12:30 PM in the U.S. Capitol to spotlight the growing public health threats posed by illegal and mass-compounded GLP-1s. To register to attend in person or virtually, visit https://ow.ly/f70B50Wq0i5
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.

Patient safety issues in the GLP-1 space this week
Tennessee Attorney General Jonathan Skrmetti and U.S. Senator Marsha Blackburn urged the Federal Trade Commission to investigate the dangers posed by counterfeit GLP-1 drugs to patients in the Volunteer State and beyond.
International News
Botulism cases continue to mount in the U.K., sentencing delayed for Canadians who used pill press to make fentanyl pills and fake ED meds.
The number of cases of botulism poisoning in the United Kingdom has risen to 38 in six weeks. The UK Health Security Agency (UKHSA) suspects the use of unlicensed Botox-like products is behind the outbreak. Victims have reported having difficulty swallowing, slurred speech, and have required respiratory support due to breathing difficulties.
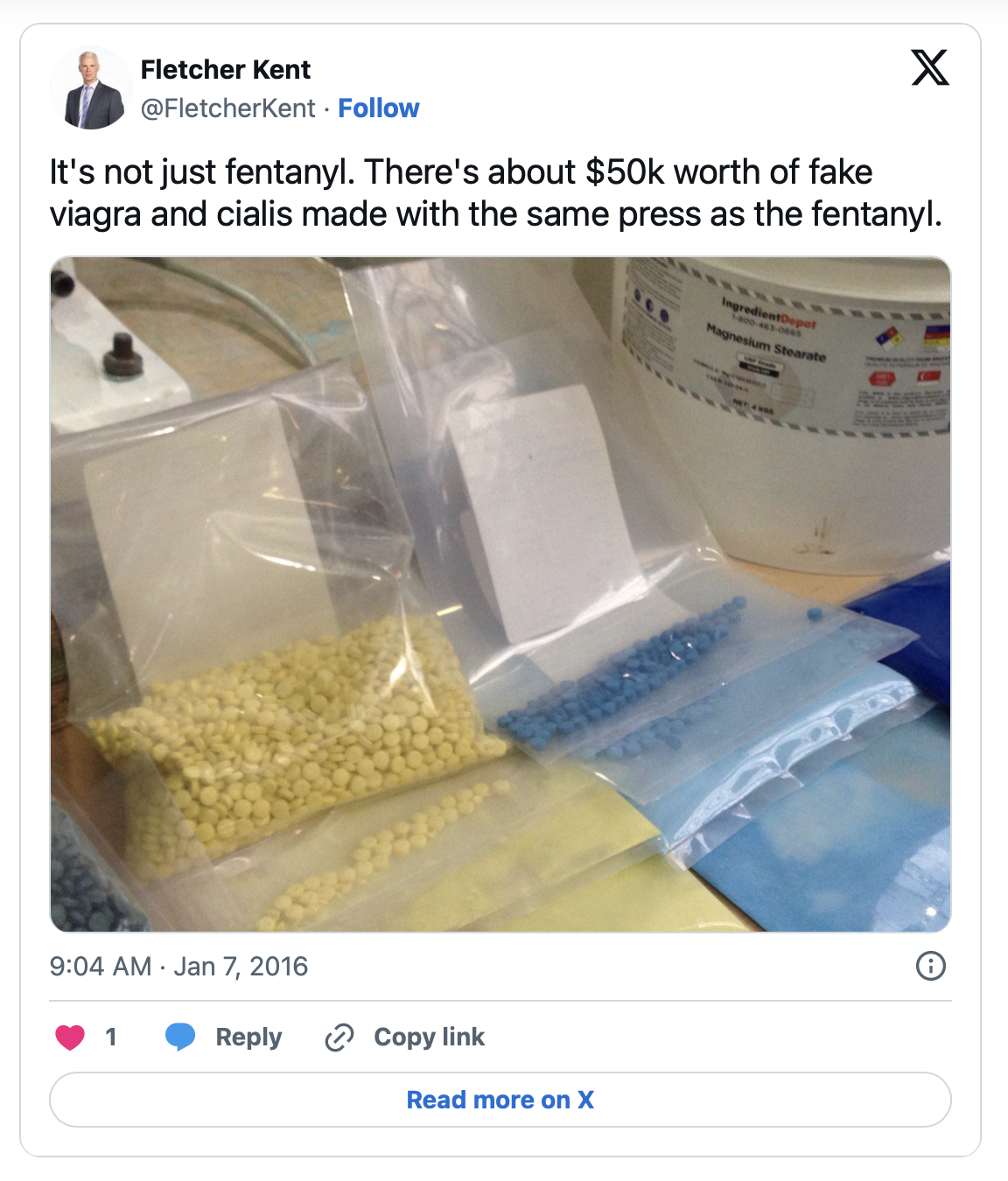
Sentencing delayed for two Canadian men in a case stemming from a New Years Eve bust in 2015 where police seized 9,000 fentanyl pills, a pill press, and counterfeit Viagra and Cialis pills. Reporting from January 2016 stated that the same pill press was used to make both the fentanyl pills and the counterfeit erectile dysfunction medications.
Authorities with Delhi, India’s health department seized suspected counterfeit drugs and medical devices from two businesses, one a dental supplier and the other a pharmacy operating at the Nigam Medical Market, a cluster of medical supply shops located across the street from a government run hospital.
Police in Uttarakhand, India arrested the owner of Dr. Mittal Laboratories, a pharmaceutical manufacturer that allegedly produced over 10 million counterfeit pills over a three-year span.