June 30, 2025: Operation Pangea XVII nets $65 million in unapproved and counterfeit medicines
Major Stories
Interpol announced 769 arrests and $65 million in unapproved and counterfeit medications seized globally as part of Operation Pangea XVII. The seizures and arrests were the largest in the operation’s 17-year history. Nervous system agents, such as medications to treat Parkinson’s, anxiety, and psychostimulants, were the most frequently seized class of drug. Medicines to treat erectile dysfunction, diabetes, and anti-smoking products were also seized.
Over the six-month period of the operation, law enforcement launched 1,728 investigations and issued 847 search warrants targeting criminal networks engaged in the illicit distribution of pharmaceutical products in 91 countries. Additionally, approximately 13,000 criminal-linked websites, social media pages, channels, and bots used to market and sell illegal or counterfeit medicines were shut down.
Domestic News
A Florida distributor received an FDA warning for dangerous DSCSA violations. Pill press seizures in Massachusetts and Georgia.
The Arkansas Board of Pharmacy used NABP's Pulse to ID the illegitimate Ozempic pen.
The FDA warned Florida-based wholesaler Sterling Distributors over significant violations of the Drug Supply Chain Security Act, including selling prescription medicines without the appropriate license, buying products from unauthorized trading partners, and failing to identify or investigate suspect products in its possession. The distributor sold counterfeit Ozempic pens to pharmacies in Arkansas and Mississippi in late 2024.
Novo Nordisk terminated its collaboration with Hims & Hers Health due to concerns that the company continues to illegally sell mass compounded semaglutide. The CEO of Hims & Hers said in a statement last week that the company will continue to market compounded semaglutide. Just four months ago, the company announced that it would stop selling compounded semaglutide products in response to the FDA ending the drug shortage.
The Iowa Board of Pharmacy scheduled a hearing on July 18, 2025 to address charges that compounding pharmacy FarmaKeio of Richardson, Texas violated provisions of the federal Food, Drugs, and Cosmetics Act. In 2022, the FDA issued a public warning about the uncertainty of the company’s sterile products. California’s Board of Pharmacy is currently weighing whether to pull the company’s nonresident license over multiple allegations.
Detectives executing a search warrant on a house in Slidell, Louisiana seized over 4,000 tapentadol pills.
Travis Sentell Howell of Baltimore, Maryland pleaded guilty to his role in a drug trafficking conspiracy that distributed cocaine in the Baltimore area. Investigators seized a pill press and pill press parts over the course of the investigation.
An investigation by state and federal law enforcement officers led to the arrest of a Medford, Massachusetts resident and the seizure of a pill press and multiple types of counterfeit prescription pills.
A federal indictment alleges that seven Georgia residents were part of a drug trafficking operation that used a pill press to make counterfeit oxycodone, Adderall and Percocet pills and sold them on darknet marketplaces. Searches related to the investigation yielded, among other things, kilograms of fentanyl powder and cocaine and a pill press with multiple die casts and molds.
Pharmacy Benefit Managers
Independent pharmacists in Ohio worry that unless a legislative solution is found, pharmacy benefit managers' under-reimbursement practices will push more pharmacies out of business and leave more Ohioans in pharmacy deserts.
A recent article about pharmacy closures in San Francisco also noted the negative impact of under-reimbursement on neighborhood pharmacies.
A group filed a lawsuit in federal court seeking to block Iowa’s Senate File 383 from going into effect. The law requires pharmacy benefit managers to reimburse pharmacies at least as much as it costs to purchase the drug.
Legislation
PSM monitors new state legislation weekly in the areas of pill presses, prescription drug affordability boards, and drug importation. Last week:
- Maine’s legislative branch passed SB 697, which directs the state’s Prescription Drug Affordability Board to examine new strategies to reduce spending on prescription drugs, including setting upper payment limits.
- Oregon’s House of Representatives held a hearing on HB 2175, which would make it a crime to possess, purchase, make, deliver, or sell a pill press or similar equipment for the purpose of making a controlled or counterfeit substance.
Patient safety issues in the GLP-1 space this week
An article in Reuters about the GLP-1 gray market interviewed Americans using the drugs, including a woman who accidentally overdosed herself with a combination of tirzepatide and as-yet unapproved cagrilintide. The piece cited PSM research about the tide of injected weight loss medicines coming into the U.S. from unregistered foreign sources.
International News
Canada is seeking public comment about changes to pill press and precursor regulations. Authorities in New Zealand and Australia reported counterfeit medicines. News about counterfeit oncology drugs in Ghana, India, Nepal, and Sri Lanka.
Canada’s Minister of Health announced a 45-day public comment period on proposed changes to how Canada regulates precursor chemicals and devices such as pill presses and encapsulators that could be used in the illegal production of drugs.
A nonprofit in New Zealand issued a public warning after it found counterfeit alprazolam pills made of tramadol for sale in blister packs.
Australia’s Therapeutic Goods Authority issued safety alerts about counterfeit semaglutide and ivermectin tablets, fake cosmetic injections, and substandard semaglutide injections.
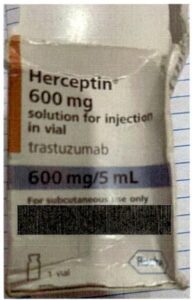
NAFDAC shared images of the counterfeit Herceptin found in Ghana.
Authorities in Ghana warned the public about a counterfeit version of Herceptin, a drug that treats breast cancer, that is believed to have entered the country from nearby Nigeria.
A family of a Nepalese woman who died from esophageal cancer in 2023 is speaking out after releasing they ordered her cancer medication, Keytruda, from a Delhi man who has been indicted in a counterfeit cancer drugs case.
The Sri Lankan government charged 12 people, including a former health minister, alleged to have purchased more than 6,000 vials of counterfeit human immunoglobulin and rituximab, a cancer medicine, for the Medical Supplies Division of the Ministry of Health. The fake treatments reached patients in state-run hospitals.
Police in Hyderabad, India arrested two individuals, alleging they were behind counterfeit cholesterol drugs seized earlier this month.

