May 27, 2025: Global effort to disrupt darknet marketplaces yields 4,400 pounds of illicit drugs
Major Stories
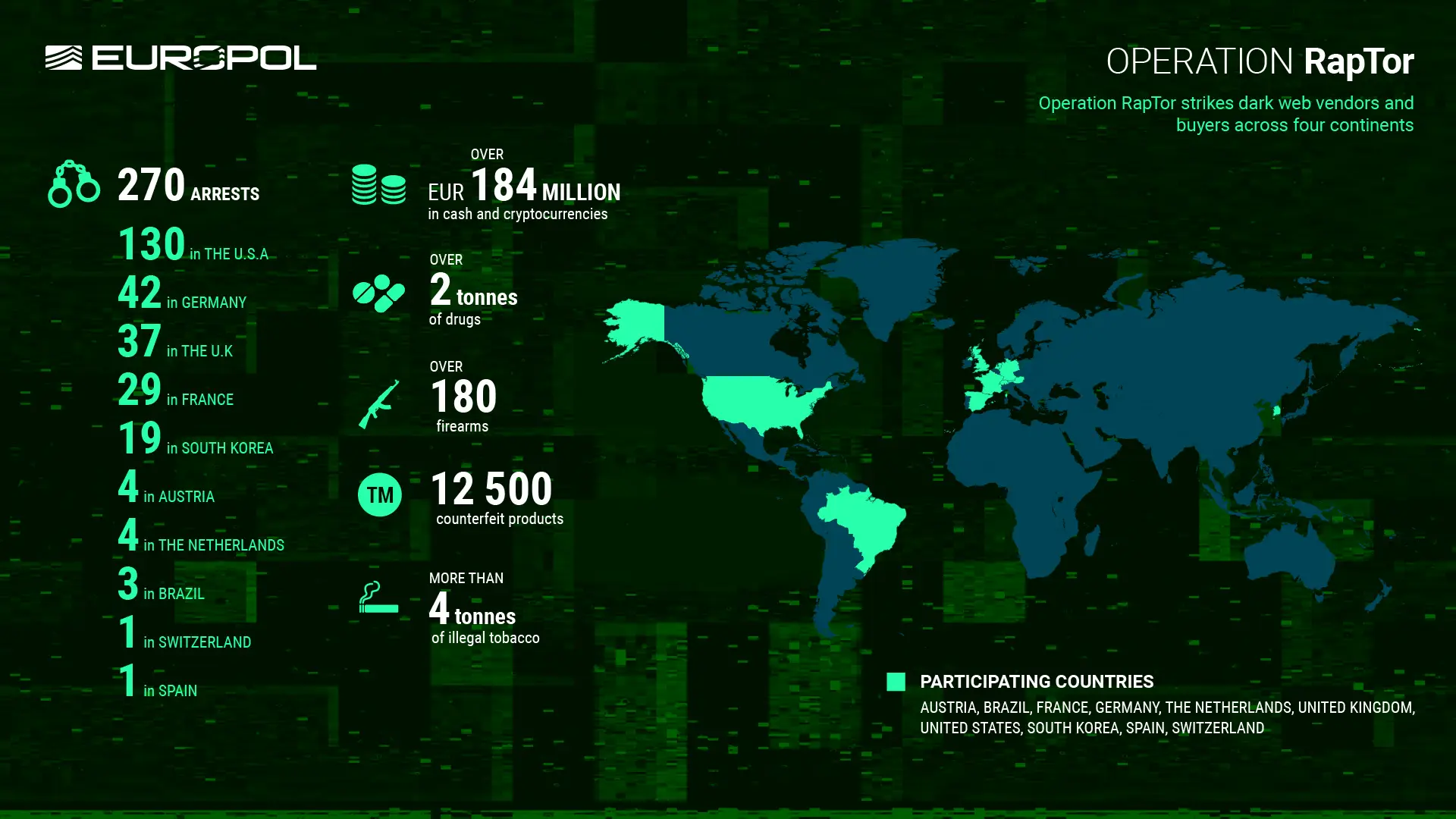
The U.S. Department of Justice announced the results of Operation RapTor, a global effort to dismantle darknet marketplaces that led to the arrest of 270 dark web vendors, buyers, and administrators and the seizure of over $200 million in currency and digital assets, 180 firearms, and two metric tons of drugs—including counterfeit prescription pills made with methamphetamine, fentanyl and other illicit substances.
Domestic News
The FDA warned a factory in China over unsanitary conditions. Two supplements were recalled because of undeclared drugs. A spa owner used fake Botox on patients.
The U.S. Food and Drug Administration (FDA) posted an April 4, 2025 warning letter it sent to Wuxi Medical Instrument Factory Co. over unsanitary conditions, including dead insects inside the tubing used to transfer active ingredients and visibly dirty packaging facilities. The agency also sent letters to two third-party testing companies in China after discovering invalid or falsified data for premarket device submissions.
Last month the White House directed the FDA to help states move forward with plans to import medicines from Canada, but Canada has not agreed to participate. In a statement to Biospace, the Canadian government said that the country’s food and drug regulations prohibit fabricators, wholesalers and distributors of prescription drugs from distributing Canadian drugs outside of Canada “if that sale would cause or worsen a drug shortage in Canada.”

Ever wonder how how federal workers protect us from dangerous medicines? (CBP)
Arizona-based UMARY USA recalled all lots and expiration dates of Unavy Ácido Hialurónico and Umovy Ácido Hialurónico. The dietary supplements, which are marketed for joint pain and arthritis, contain undeclared diclofenac, dexamethasone and omeprazole.
Prosecutors in Houston, Texas have charged an unlicensed med spa owner who allegedly injected her patients with counterfeit Botox that caused physical injuries.
Patient safety issues in the GLP-1 space this week
Connecticut’s attorney general sued Florida-based Triggered Brand for allegedly selling “research grade” GLP-1 peptides directly to Connecticut consumers without prescriptions or medical oversight and issued a civil investigative demand to Made In China, a Chinese international trade platform that sells “research grade” GLP-1s to U.S. customers. The AG also warned weight loss clinics and medspas that marketing compounded GLP-1 drugs is no longer legal.
International News
News about counterfeit medicines in Cambodia, India, Pakistan, Saudi Arabia and Vietnam.
After three years, police in Delhi, India arrested a fugitive who was involved in a counterfeit medicine operation that distributed fake cancer drugs in Kolkata and Bangladesh.
Authorities in West Bengal, India banned 137 medicines that had failed quality standards and urged drug sellers to ensure that their medicines are legitimately sourced by verifying wholesalers’ licenses and goods and services tax numbers.
Saudi Arabia's Food and Drug Authority fined a pharmaceutical warehouse in Riyadh the equivalent of $30,000 for possession of pharmaceutical products sourced from an unlicensed entity, unsafe shipping of medicines, and trading in counterfeit pharmaceutical products.
Cambodia, Pakistan, and Vietnam also issued warnings about counterfeit medicines.