August 4, 2025: Pennsylvania pharmacy owner indicted and 14 licenses revoked in Ohio
Major Stories
PA pharmacy distributed unregulated HIV meds to patients, OH BOP revokes licenses, and more.
An investigation into a pharmacy in Pennsylvania resulted in criminal charges. The state alleges that the defendant ran a multi-million dollar scheme to dispense unregulated HIV medications to patients. The investigation revealed that the medications were purchased from a source other than the pharmacy’s legitimate wholesale suppliers, with employees reporting that the bottles felt “sticky,” as if labels had been removed.
David Sunday, Jr., the Pennsylvania Attorney General, stated, “The investigation revealed that nearly 100,000 tablets of HIV medications dispensed at the pharmacy were not obtained through documented purchases from authorized distributors.” The pharmacy is no longer in business.
The Ohio Board of Pharmacy published a list of 14 medical establishments in the state that have had their licenses to distribute medicine revoked so far this year. The majority of these establishments are medical spas, one of which PSM reported on earlier this year.
Politico’s AgencyIQ, a regulatory intelligence service specializing in life sciences and chemicals, referenced PSM’s most recent Fishy Freight report in its August 1, 2025, newsletter:

Teen Vogue released an article examining the dangers patients face when supplements are purchased from unreliable sources. One of the cited incidents involved a weight loss supplement that contained two undeclared active pharmaceutical ingredients. One of the ingredients – sibutramine – is banned in the U.S. because it has been linked to heart problems. The woman who had purchased the supplement only learned this information after seeing her doctor due to several alarming symptoms, including a racing heart, constant nausea, and spasming fingers.
The Southern Nevada Health District and the Nevada Board of Pharmacy are investigating incidents of hospitalization allegedly tied to peptide injections received at a conference in Las Vegas. At least two attendees required transport to a local hospital, where one required ventilation to assist with breathing, with reports of potentially as many as seven individuals needing to be hospitalized.
Domestic News
DOJ indicted a NV resident, pill press cases in WA and MS, four warning letters, and more.
Lawyers with the Department of Justice (DOJ) allege that a Nevada man made millions of dollars importing and selling pills marketed as all-natural erectile dysfunction medications at smoke shops, convenience stores, and online. According to the DOJ, the man purchased the pills from a company in India, and the pills contained undeclared active pharmaceutical ingredients.
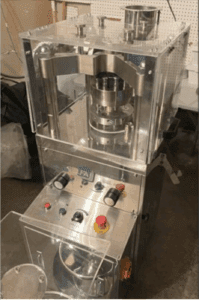
Image: pill press seized in WA.
Source: DOJ
A man in Auburn, Washington, received an 11-year prison sentence for converting his garage into a fentanyl pill lab. The defendant lied about operating a vitamin manufacturing business in order to purchase an industrial pill press.
A federal jury convicted a Meridian, MS resident in a drug trafficking case. According to law enforcement, the defendant used two pill presses to manufacture counterfeit pharmaceutical pills that contained methamphetamine, fentanyl, and cocaine.
The U.S. Food and Drug Administration issued warning letters to multiple websites for the unlawful sale of unapproved and misbranded drugs to United States consumers over the Internet:
- www.d-pharmacy of Singapore
- www.mysteroidsmarket.com of Israel
- www.portmeds.com of the United States
- www.plakini-pharma.com of Malaysia
Senators Ted Budd (R-NC) and Martin Heinrich (D-NM) introduced an amendment to the 2026 Agriculture, Rural Development, Food and Drug Administration, and Related Agencies Appropriations Act to ensure that the FDA is able to use its existing regulatory authority to prevent illicit weight loss products from getting into the country.
The Department of Health and Human Services (HHS) started accepting applications for a new 340B pilot program that would replace upfront discounts with what would essentially be a post-purchase rebate given to covered entities. Post-purchase rebates would only be issued after data has been submitted to show the medication was administered to the intended program participants. The pilot program will only be operational for ten medications and aims to start on January 1, 2026.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
Patient safety issues in the GLP-1 space this week
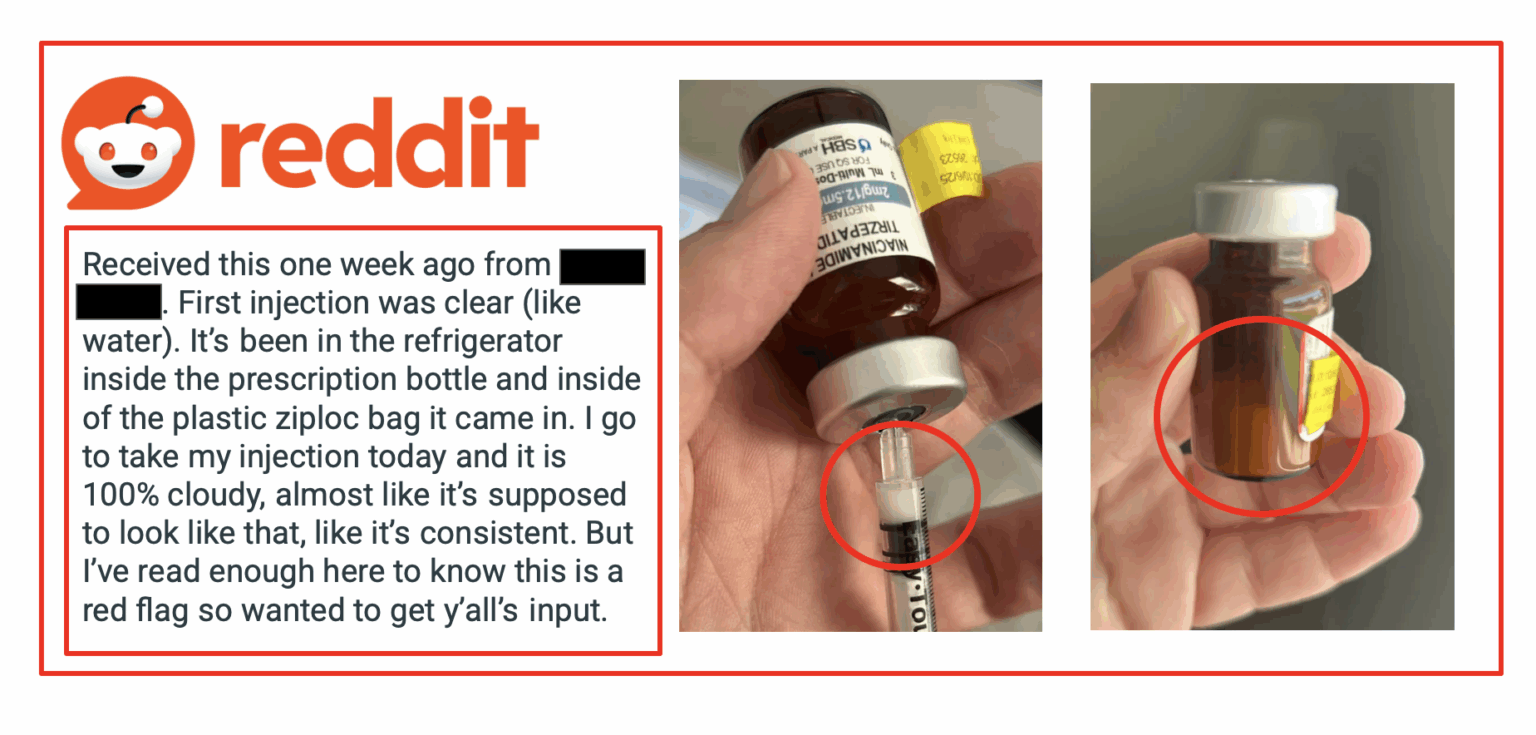
On August 1st, a Reddit user was seeking advice and shared images of the recent shipment of tirzepatide that had changed color:
International News
Health Canada warning about unlicensed peptides and medicine counterfeiters busted in India and Uzbekistan.

Image of cooler containing peptide vials.
Source: Health Canada
Health Canada issued a warning about a peptide company selling unlicensed products to customers via the Internet. The warning listed 40 different unapproved peptides for sale on the company’s website.
The Uttarakhand Special Task Force in India has arrested the owner of a medical store for his role in a counterfeit medicine case involving life-saving, brand-name drugs. The task force alleges that the counterfeiters used an ambulance to sell the fake medicine.
Authorities in Delhi, India, busted a counterfeit medicine group operating out of an apartment in the Paschim Vihar area and involved fake versions of medications made by several legitimate manufacturers.
Law enforcement and customs officials in Uzbekistan disrupted a counterfeit medicine operation, uncovering the facility where the fake medicine was being produced.
