May 4, 2025: New study suggests more adverse events associated with compounded GLP-1s than standard formulations
Major Stories
New research shows a correlation between compounded weight loss and diabetes injectables and increased adverse events. Gilead Sciences is suing another New York City pharmacy that allegedly sold counterfeit HIV medicine.
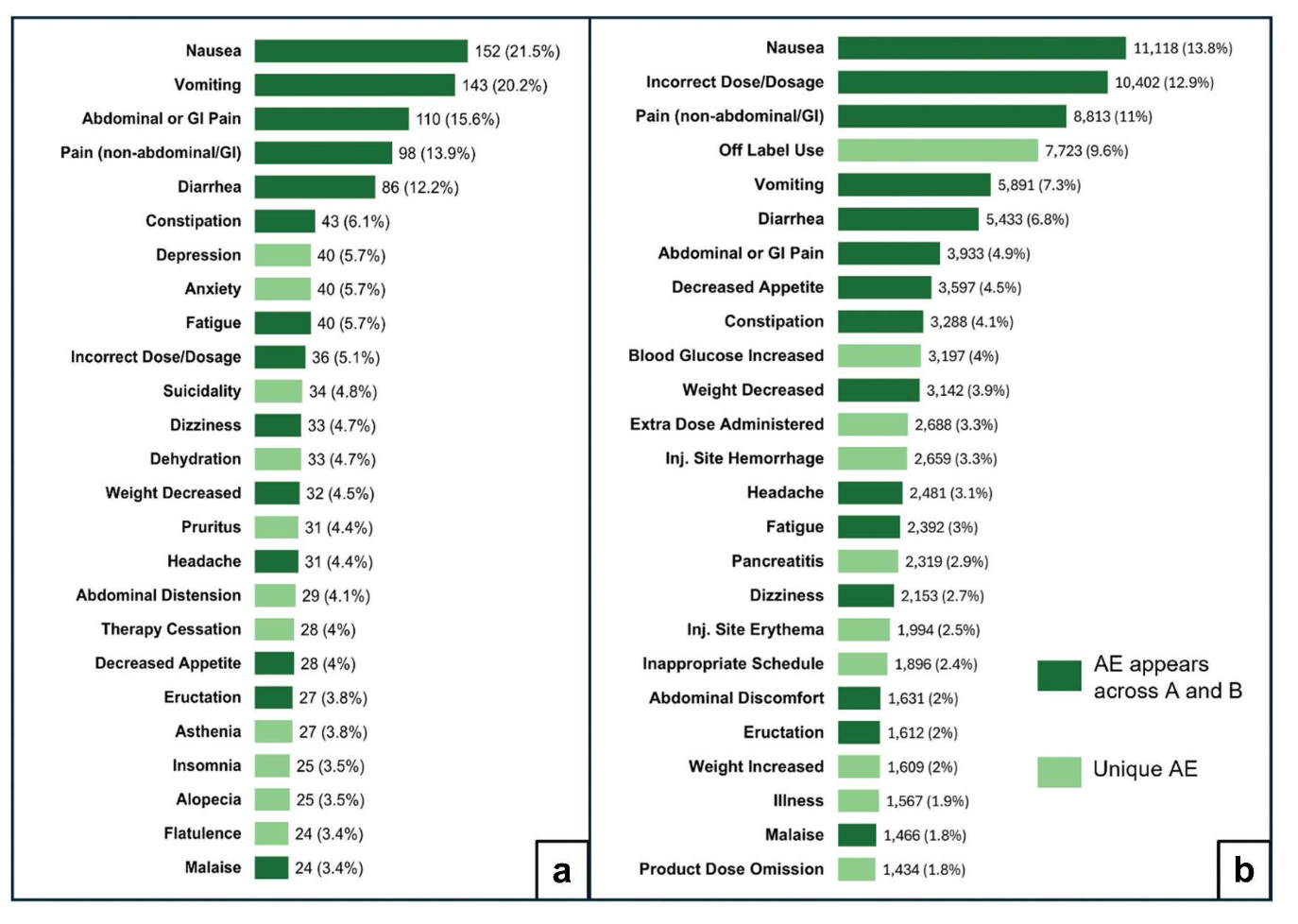
A new paper published by pharmacist and PSM board member Dr. Kenneth McCall suggests that compounded GLP-1s pose may more safety issues than their manufactured counterparts. After reviewing data submitted to the FDA’s Adverse Event Reporting System between 2018 and 2024, McCall and his coauthors found that compounded GLP-1 medicines were correlated with a greater likelihood of abdominal pain, nausea, diarrhea, gallbladder inflammation, and suicidality compared to standard formulations, as well as more frequent hospitalization for those adverse effects. Researchers also found a higher incidence of prescribing and preparation errors related to the compounded medications.
A U.S. District Court in New York unsealed a March 2025 complaint filed by Gilead Sciences against a pharmacy in Queens, New York alleged to have sold counterfeit Biktarvy, an HIV treatment, and refused to disclose its source. The counterfeit, which came to Gilead’s attention because of a patient complaint, came with a fake label that seemed to have been applied by machine, which suggests a high volume counterfeiting operation. Read the complaint here.
Patient safety issues in the GLP-1 space this week
Patients seeking alternative sources for their semaglutide and tirzepatide injections need to know that the drugs these pharmacies sell to Americans are not the same as the ones Canadians take, and neither Health Canada nor the FDA are regulating them.
Domestic News
The FDA acted to protect Americans from dangerous medicines. News involving pill presses in four states.
The FDA warned two pharmaceutical manufacturers that they had violated Current Good Manufacturing Practice regulations. Among other things, the companies failed to adequately test medicine components for diethylene glycol (DEG) or ethylene glycol (EG) contamination before using them. DEG and EG are industrial solvents that have made their way into medicines made with glycerin or propylene glycol with deadly results; cough syrups adulterated with DEG killed 300 children in Gambia, Indonesia and Uzbekistan in 2022.

The FDA also warned Amazon to stop selling lidocaine creams that exceed FDA-approved concentrations for over-the-counter products. The agency warned consumers about the dangers of products like these in March 2024.
News about Prescription Drug Affordability Boards (PDABs)
Maryland's and Colorado's PDAB stakeholder councils met on April 28 and April 24, 2025. Colorado's stakeholder council was informed that the creation of an upper payment limit (UPL) for Enbrel was delayed until the May meeting because of a data categorization error that swapped Medicaid and Medicare information for 7% of the of the pharmacy claims in the Colorado All Payers Claim Database Data. Maryland's stakeholder council heard an overview by Brian Link of Three Axis Advisors about drug pricing and the supply chain. Video is available here.
The Alcorn County Sheriff’s Office arrested a store owner in Corinth, Mississippi and seized products made of kratom and tianeptine as well as more than 3,500 unapproved sildenafil (Viagra) tablets.
Arizona-based Health Fixer recalled dietary supplements because they are adulterated with sildenafil and related erectile dysfunction drugs.
Timario Gayton and Quonzy Hope, of Rock Hill, South Carolina, each received a 15-year prison sentence for fentanyl distribution. Law enforcement seized 29 kilograms of fentanyl along with, other illicit drugs, several pill press machines, cash, and drug paraphernalia during the investigation.
Saugus, Massachusetts resident Aaron Lenardis received a 15-year prison sentence for fentanyl and methamphetamine distribution after he and co-conspirator Charles Bates made at least 136,000 counterfeit pills containing methamphetamine. A search of his house in October 2022 yielded firearms, an industrial pill press, “M30” stamps commonly used to make fentanyl pills, methamphetamine and fentanyl and more than two kilograms of pills.
In Florida, the Pinellas County Sheriff’s Office busted a drug trafficking ring, arresting 19 people and seizing pounds of illicit drugs, including fake oxycodone pills made with fentanyl and methamphetamine pills disguised as Adderall, a pill press, firearms, and $14,000 in cash.
Police in Akron, Ohio seized methamphetamine, fentanyl and a pill press in a joint operation with the Federal Bureau of Investigation.
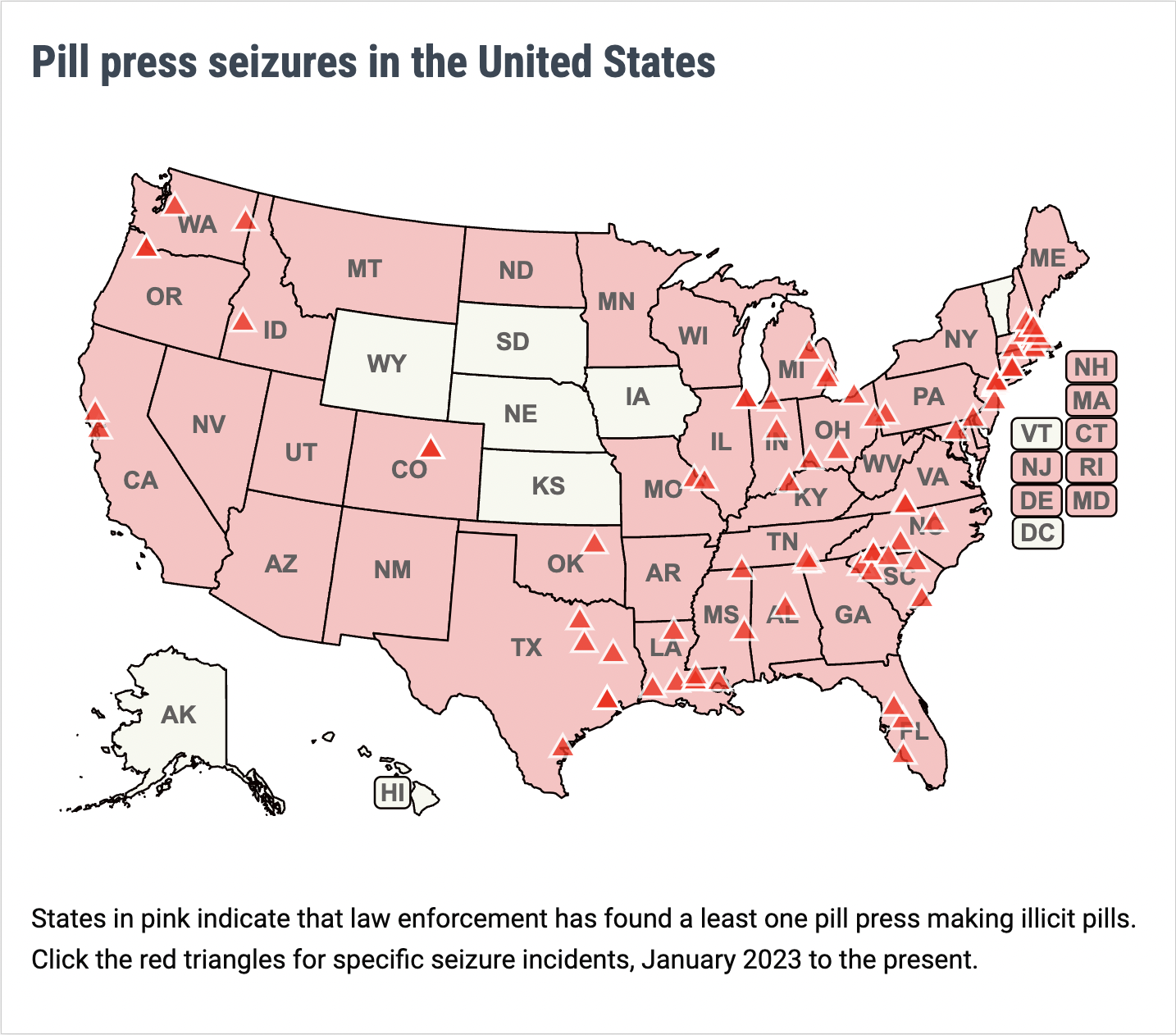
For more information about policy issues around pill presses, visit PSM's pill press page.
International News
Counterfeit and substandard medicines reported in the U.K., Pakistan, Cameroon and the Central African Republic.
Britain’s Medicines and Healthcare products Regulatory Agency’s Criminal Enforcement Unit seized hundreds of thousands of doses of controlled drugs such as opioid painkillers and anti-anxiety medicines from 22 properties across West Midlands, Greater Manchester, Staffordshire and Merseyside.
The Drug Regulatory Authority of Pakistan (DRAP) issued an alert over a counterfeit version of Rhophylac 300, an injection that suppresses autoimmune reactions in Rh-incompatible pregnancies and blood transfusions.
The WHO issued a medical alert about counterfeit amoxicillin circulating in Cameroon and the Central African Republic.