Prescription Drug Affordability Board Activity through April 16, 2025
Activities Summary
Colorado: Colorado's PDAB met on April 11, 2025, where it addressed an error in All-Payer Claims Database data and how it affected affordability review information for Enbrel, Cosentyx and Stelara.
Maryland: Maryland's PDAB did not meet in April.
Oregon: Oregon's PDAB met on April 16, 2025 to review a draft of the generic drug report and to discuss the timeline, process and voting methodology for affordability review determinations.
Washington: Washington's PDAB did not meet in April. The next meeting will be May 21, 2025.
Activity by State
Colorado PDAB Meeting April 11, 2025
Board Member & Director Updates
The PDAB has a new Prescription Drug Affordability Director, Sophie Thomas, MPH. She is overseeing prescription drug affordability programs and PBM enforcement. Lila Cummings is becoming the Deputy Commissioner of Health Policy.
Enbrel Upper Payment Limit (UPL) rulemaking: The board held a discussion about All-Payer Claims Database (APCD) data validation in lieu of the UPL rulemaking hearing. During the May 23, 2025 PDAB meeting, the board may vote on whether or not to begin UPL rulemaking for Enbrel.
Amgen v. Mizner, et al. update – The court dismissed the case on procedural grounds, specifically that Amgen did not have standing to bring the case. However, Amgen will appeal in federal circuit court.
The board has published cost benefit analysis and regulatory analysis reports for the proposed Enbrel UPL rule and welcomes stakeholder comments. Sign up forms for written and oral comments will be posted on the PDAB page “soon.”
Addressing the significant error in the previously provided APCD data: Explanation
7% of pharmacy claims in the Colorado All Payers Claim Database Data (APCD) were mislabeled by a PBM. Explanation follows.
Overview of data miscategorization
- A PBM miscategorized its commercial and Medicare claims data
- For pharmacy claims, the PBM mislabeled commercial claims as Medicare claims, and vice versa
- These pharmacy claims accounted for 7% of total pharmacy claims in the APCD
- Even though the categorization of data changed, utilization still occurred on Medicare claims
- However the DOI does not have access to all Medicare Claims, just access to Medicare Advantage
- As a result, corrected data shows up as changes in APCD-related utilization and expenditure data
- The data miscategorization will not affect the data presented to the board by staff during the UPL rulemaking.
Revisit data sources used by the board
- Colorado All-Payer Claims Database is not the only data used by the board
- Other sources:
- First Data Bank/Analysource
- SSR Health
- FDA Website
- DA Orange & Purple books
- Center for Medicare and Medicaid Services
- Peer-reviewed health outcomes research
- CDC Social Vulnerability Index
- Bureau of Labor Statistics
- Surveys
- Voluntarily Submitted Info
- Stakeholder Meetings
Data points impacted due to the PBM’s data errors in the APCD
- Number of patients using prescription drug per annum
- Average wholesale acquisition cost (WAC)/course of treatment
- Average amount paid by the patient and payer per person/year
- Average amount paid for the drug by the payer/per/year
- Average amount paid for the drug by the patient/year
- Total paid by payers & patients within one year
- Total paid by all patients within one year
Staff revised the incorrect data. The information will be corrected in tables, and represented with teal bars. The original incorrect data will appear in purple headed tables.
Addressing the data error: Revisions for Enbrel, Cosentyx and Stelara
Staff revised the incorrect data. The information will be corrected in tables, and represented with teal bars. The original, incorrect data will appear in purple headed tables.
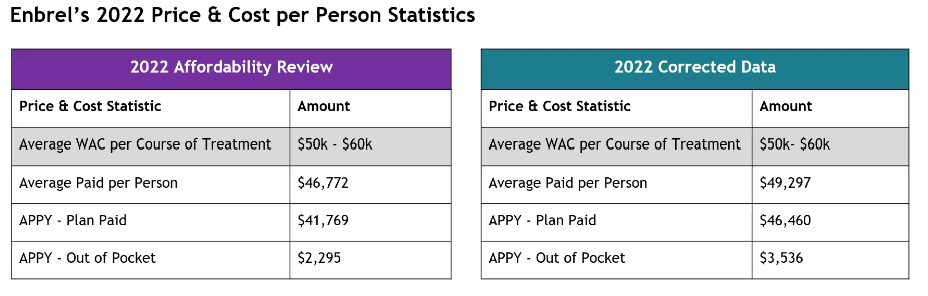
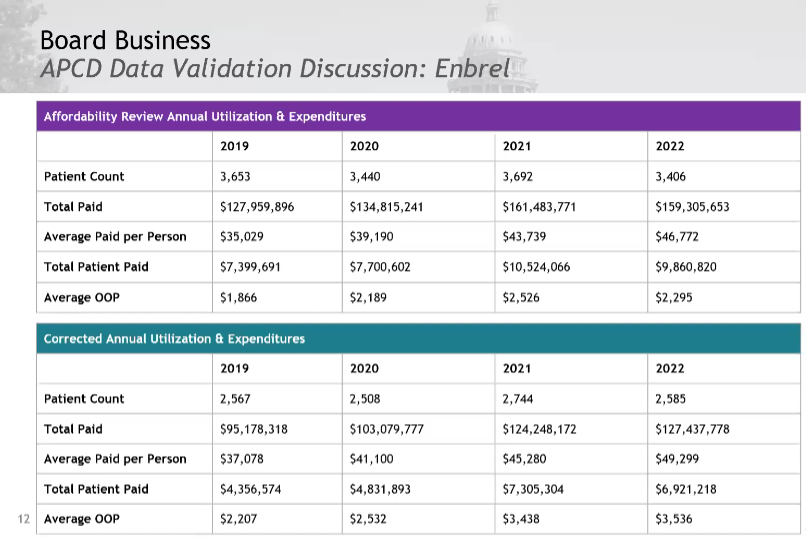
Enbrel Validated Data Presentation and Discussion
The costs are higher in the corrected data.
Notably, the correct data shows a decrease in utilization. The total patient paid (total patients in the state) is approximately 2/3 of the original number, but the average paid per person is higher. These amounts don’t include copay cards.
The Board’s conclusion is that Enbrel is even more expensive than originally thought. The board sees this population of 2,500 as a significant patient population for the state which has 6 million inhabitants. The board asked for percentages and directional arrows to change direction in average WAC. Staff will add an addendum to report with corrected data and update data to allow the board to confirm Enbrel’s eligibility. The board asked that this be attached to the executive summary.
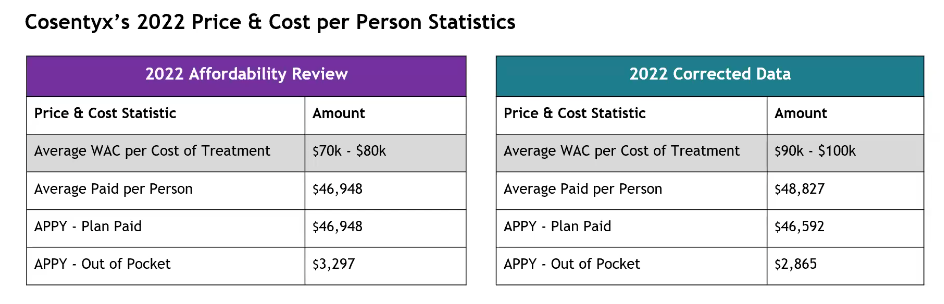
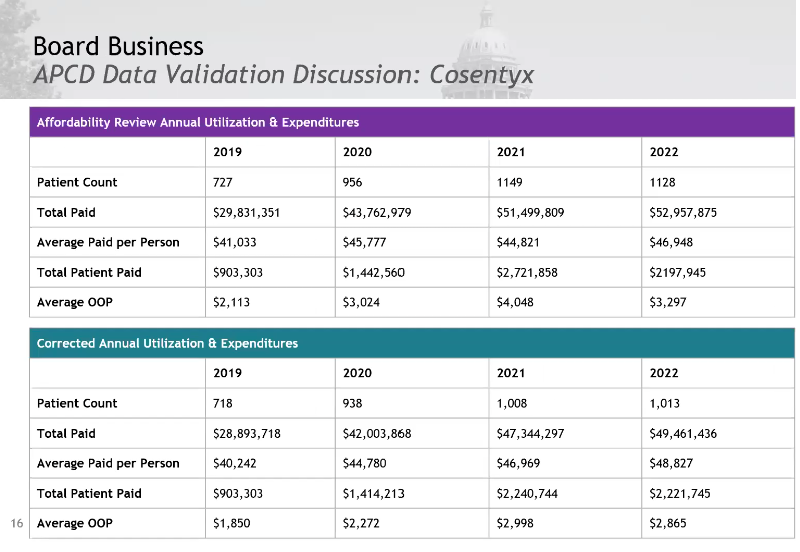
Cosentyx Validated Data Presentation and Discussion
This chart is wrong. The purple average paid per person and APPY – plan paid numbers are incorrect. The average paid per person was 46,948; APPY, 44,963. The other numbers are correct. The board asked why the average WAC per cost of treatment increased so significantly.
Average out-of-pocket and plan-paid numbers decreased, while the average WAC increased.
Staff will provide more information on why the band of prices went up so significantly and add an addendum to the report with the corrected data and updated data to allow the board to confirm Cosentyx’s eligibility. The board asked that this be attached to the executive summary.
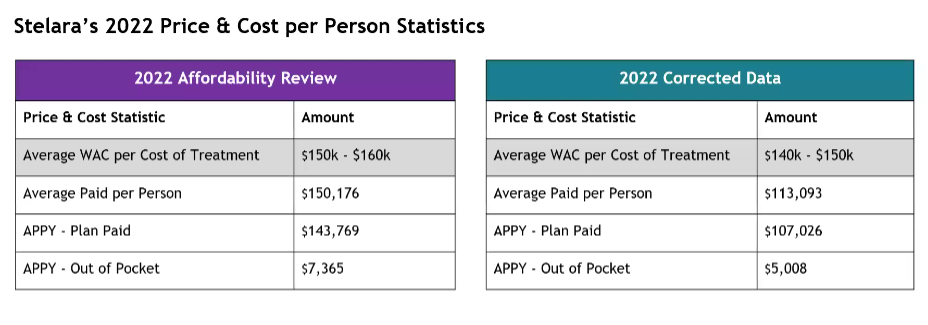
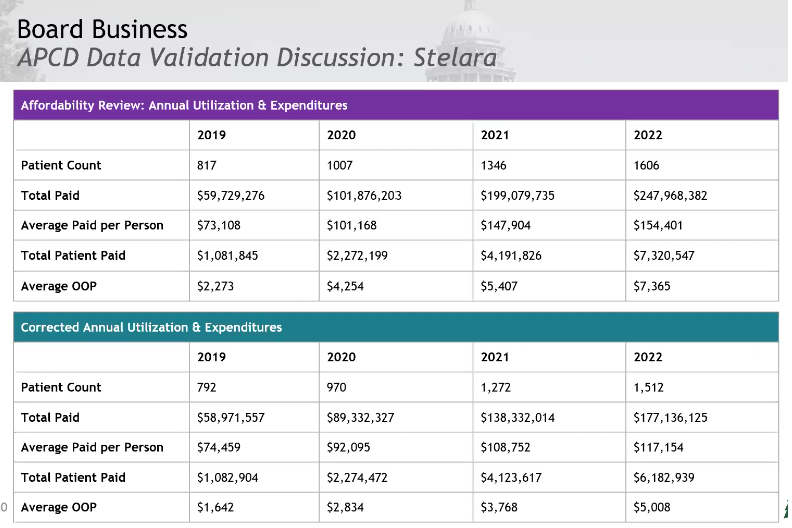
Stelara Validated Data Presentation and Discussion
The band of WAC went down when corrected. The average paid per person decreased significantly, the plan paid dropped and the average out of pocket went down.
Staff will provide more information on an initial loading dose and how that might impact these numbers. They will also add an addendum to the report with the corrected data and update the data to allow the board to confirm Stelara’s eligibility. The board asks that this be attached to the executive summary.
Public comment
Written comments were submitted by LMDD Colorado, HealthHIV, CANN and Act Now. They can be found here.
Oral comments were provided by:
Amanda Boone, a cystic fibrosis advocate, was concerned that PBMs are not providing accurate data. Next Wednesday there will be a hearing about PBM drugs and drug rebates in the cost of prescription drugs. If we don’t fix PBMs and drug rebates we won’t lower out of pocket costs for patients or payers.
Tiffany Westrich Robertson, Executive Director of AiArthritis, was concerned with looking at the out-of-pocket patient cost of Enbrel. If including copay assistant programs those numbers should be closer to zero. Is the data provided including Medicare? It needs to be separated before making a UPL ruling.
Sophia Hennessy, Colorado Consumer Health Initiative, asked the board to start the UPL process for Enbrel.
Bridget Serritt of Advocates for Compassionate Therapy Now was concerned about patient out-of-pocket costs. She wanted to know what effect CMS reviews will have on board’s decisions and pointed out that the cost benefit analysis didn’t actually have a cost benefit analysis.
Evan Slater, a Colorado pharmacist, said that the analysis needed to include copay cards and assistance to address patient costs. Payers have shifted costs to patients, which is reflected in the tables, as you see greater costs to patients over time, which is then picked up by manufacturer copay cards, causing a feedback loop that increases prices.
Staff are addressing inclusion of Medicare data. They will separate out commercial vs Medicare for May. The board asked for copay card data and info from payers.
Approve Revised PDAB Policy and Procedures: Policy No. 4 (Affordability Review Policy and Procedure)
The board reviewed the policy in January. Minor changes were made and reviewed previously. The changes required that
- any conflicts of interest regarding technical information on specific drugs
- staff include estimates that separate 340b discounts from negotiated rebates to the extent that data is available,
- staff will collect information about patient assistance programs, and related data while compiling support evidence for affordability review.
The board voted unanimously to approve the revised PDAB Policy and Procedures: Policy No 4.
Stakeholder Workgroup Discussion
The board needs more info to understand conflicts of interest. They want information from under-insured patients and subspecialists who prescribe these medications. The board pointed out that they read all the commentary, and want to acknowledge people who submit comments. They want to ask PDAAC how they can get better stakeholder discussion.
Public Comment
Jennifer Reinhardt, a parent of a child with a rare disease, said it was important to maintain access to drugs. She was concerned with QALY data and with rare disease patients. PORTAL shouldn’t be used because they use QALY data. There should be a patient or caregiver on the board.
Amanda Boone, cystic fibrosis advocate, recommended that the PDAB come to stakeholder engagement sessions and put a patient on the board. She asked why the analysis didn’t include operating costs and noted that the program hasn’t saved the state any money.
Amy Goodman of Colorado Bioscience noted that the board picked the initial five drugs for review based upon the APCD data on the number of patients and the total amount paid. The board should go back and reexamine everything now we know that data is flawed.
Bridget Serritt of Advocates for Compassionate Therapy Now, agreed with previous commenters. The process needs more input from stakeholders and needs to connect with the PDAAC. She believes patients can be a good source of knowledge on this topic.
Tiffany Westrich Robertson, CEO, AiArthritis, urged the PDAB to lean on the patient community to help them. There should be funding conflict disclosures with payers as well as patient groups, groups like Arnold Ventures and Commonwealth.
Harry Gawanter, Board Member of the Let My Doctors Decide Action Network suggested that the board should have Medicaid send out requests, that pharmacies post QR codes on site. Physicians don’t know about the PDAB. Reach out and have them help you get information. Include data about copay accumulators, maximizers, payment assistant programs and formulary tiers to figure out actual patients’ out of pocket costs. Set a UPL for patients, not for list prices.
Emily Zadvorny, Executive Director, Colorado Pharmacists Society, said that most pharmacists don’t know the PDAB exists. She suggested that they formalize working with professionals through associations because clinical experts don’t know about this process. DOI could work with DORA to send communication to all licensed professionals.
The next meeting is May 23, 2025 at 10am MT.
Oregon PDAB meeting, April 16, 2025
Executive Director's Report
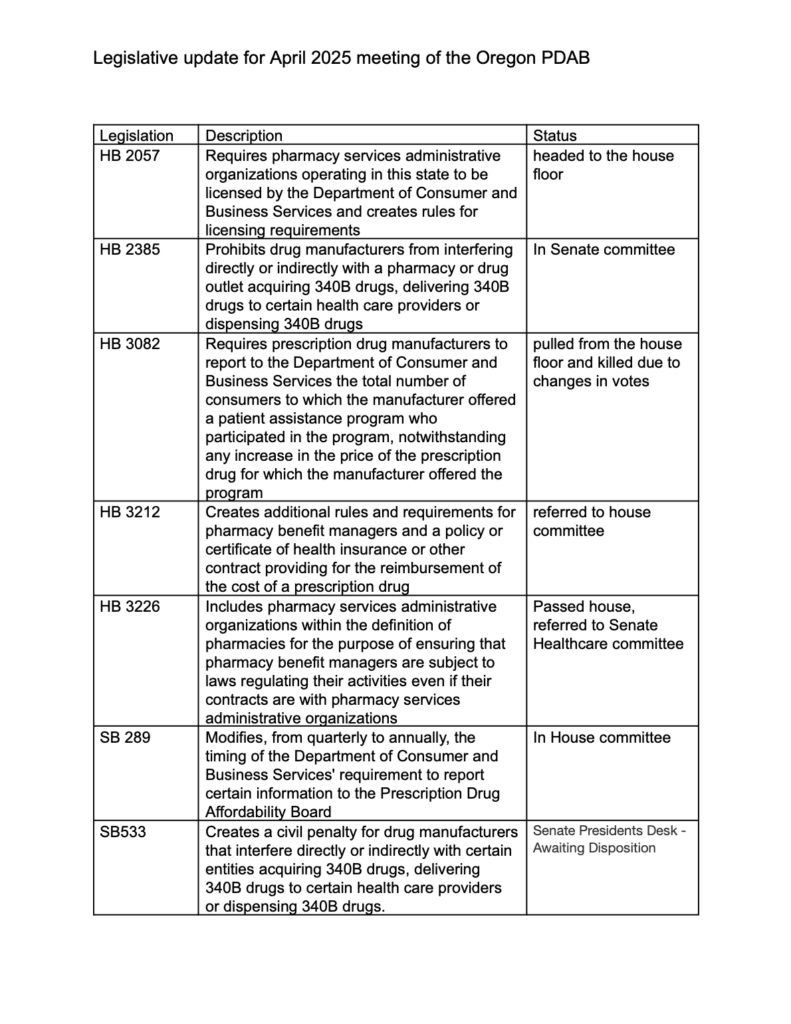
Informed members that SB 289 had left the Senate and was being considered by House Health. The bill would change the PDAB’s number of affordability reviews to “up to nine drugs” rather than fixed at nine, remove the Department of Consumer and Business Services requirement to provide the PDAB with a list of drugs that are collected by the drug price transparency program and fold a separate report on generic drugs into the annual affordability review.
A letter from the National Association of Attorneys General, on behalf of a bipartisan coalition of 39 state and territory attorneys general, urged congressional leaders to pass an act prohibiting pharmacy benefit managers (PBMs) from owning or operating pharmacies.
Robert Judge has submitted a resignation after the June 2025 meeting. The board will have a vacancy posting up through June 6, 2025. The position requires a clinical medicine or healthcare economics background.
The board is still seeking sources for commercially available data on drug pricing.
Board member John Murray was congratulated for working with the Oregon State Pharmacy Association to increase access to pharmacy services through medication lockers in rural areas.
Legislative update by Jesse O’Brien, Policy Manager for Division of Financial Regulation
The legislative director provided an update on related bills in Oregon. The following are only the bills that are currently active.
Public comment
Among the public comments, Sanofi wrote to say that Dupixent is an FDA Orphan Drug. The Society of Medical Oncology Oregon is worried that UPLs for cancer drugs may disproportionately impact Oregon cancer patients, saying in part, “We are concerned that reimbursement for a physician-administered drug with a UPL will fail to cover costs incurred for procuring, storing, and handling highly toxic agents. Medicare and private market reimbursement for physician administered drugs includes payment to cover costs associated with drug treatments in physicians’ offices. Without such payment for drugs subject to a UPL within state regulated plans, oncology practices could face a financial burden that puts them at risk of closure and patients could face delays in care or have to travel further for treatment.” The full list of public comments is available here.
Oral comments:
- Nathan Sauser, atopic dermatitis patient and Dupixent user, cost prohibitive, however he used a co-pay program, found it absurd.
- Lorren Sandt, Executive Director of the Caring Ambassadors Program, was concerned about the data collection process and timeline.
- Michiel Peters, Global Coalition on Aging, worried that UPLs may hinder innovation in new drugs, and wants HIV drugs excluded from cost reviews.
- Lisa Bozinovic, Oregon Biosciences Association, expressed concerns with methodology to determine UPLs.
- Dharia McGrew, PhRMA, was concerned with methodology to determine UPLs based upon “personal opinions of board members.” Asked the board to stick to facts, and noted that the proposal to review 8 drugs a month didn’t seem consistent with board experience, when 3 drugs was challenging.
Board review of draft generic drug report
After a closed executive session for legal advice, the board reviewed a draft of the generic drug report (available in meeting materials). A redline will be available after the meeting (Not yet posted.)
Page 6 – Addition of biologics (Hartung noted it was out of place.) Staff will work to place it appropriately.
Whitlock responded that this was the analysis based upon the carrier data 2021-2023, and used the drugs that were most costly over that timeframe. Hartung asks for clarification on how the 9 drugs are identified in the paragraph.
Hartung responded that DEA puts quotas on ADHD medication manufacturing and needs adding. Kennedy states this is a supply and demand based upon ADHD drugs being the fastest growing drug demand in Oregon. Whitlock asked for supporting documentation for all statements.
Attempt to define “multi-source drug.” Wanted to strike the red section.
Hartung stated that the headline says “Medicare and Medicaid” but it appears to not include Medicare. He asked for nuance related to Medicaid drug pricing for branded vs. generics.
Concern that tariffs are actually relevant.
Bailey suggested changing heading to PBM affiliated GPOs since no other kind of GPO is mentioned.
The Board moved back into an executive session.
Board discussion of timeline, process and voting methodology for affordability review determinations
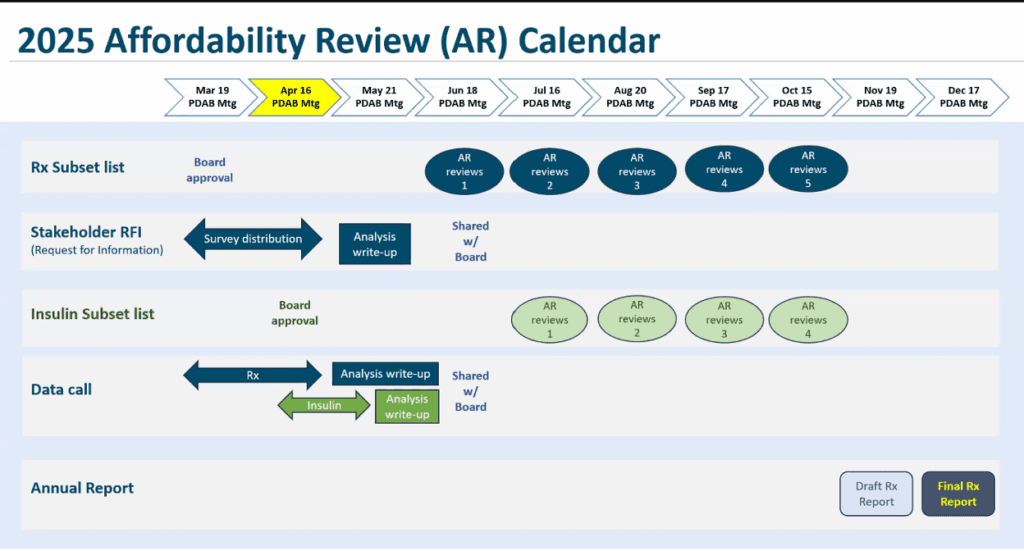
Presentation: 2025 Affordability Review Roadmap (available in meeting materials).
Review of timeline by month
- April: Set-up for Affordability Reviews (AR)
- Board consideration of a second review of the subset list in June to determine if any additional drugs should be removed from AR
- How to review information to remove additional drugs? -Consider sending a survey to board members on the top 10 drugs they want to review and compiling a list of the most requested.
- Discussion
- When should public comment be provided for each drug under review?
- Timing of voting to identify the nine drugs and at least one insulin product
- Options to vote to identify nine drugs and at least one insulin product
- When should the Board vote to identify a drug or insulin product that may cause an affordability challenge?
- Vote on each drug at the meeting it is presented or on each drug at the end of all the reviews?
- What should be the order of the drug review? Most costly, most costly by utilization, alphabetic, or other?
- Does the board want to select the Rx independently and then discuss the top-rated ones?
- May: Board meeting dedicated to consumer and supply chain feedback.
- June: In June, the board can remove drugs from the list of 27 by reviewing information about each drug on the AR sublist and determining whether it can be removed. If no drugs are removed, the addition of the selected insulin products may lead to a review of approximately eight drugs each month.
- July to October: Review of up to eight drugs each month. The board will discuss material packets for selected drugs. There will be public comment time for each Rx being reviewed. Board to initially determine whether comments will be for each drug or have one public comment time for all drugs and if that time should be at a different time than the regular public comment time.
- November: Identify nine drugs and at least one insulin product that may create affordability challenges to Oregon's healthcare system or patient out-of-pocket costs.
- December: Approve final drug report.
Additional discussion Items
What information does the board want to receive for the June 18 meeting?
The board discussed whether or not the Latin community is sufficiently engaged and whether to leave the response window open longer. Executive Director Magrish pointed out that the Latin community has responded more to social media than other groups.
Five board members want to close the response window on the original date, believing that outreach has been extensive.
The May meeting will be about staff answering the board's questions.
Board review of data sets and OAR 925-200-0010 criteria to select subset of insulin products for affordability reviews
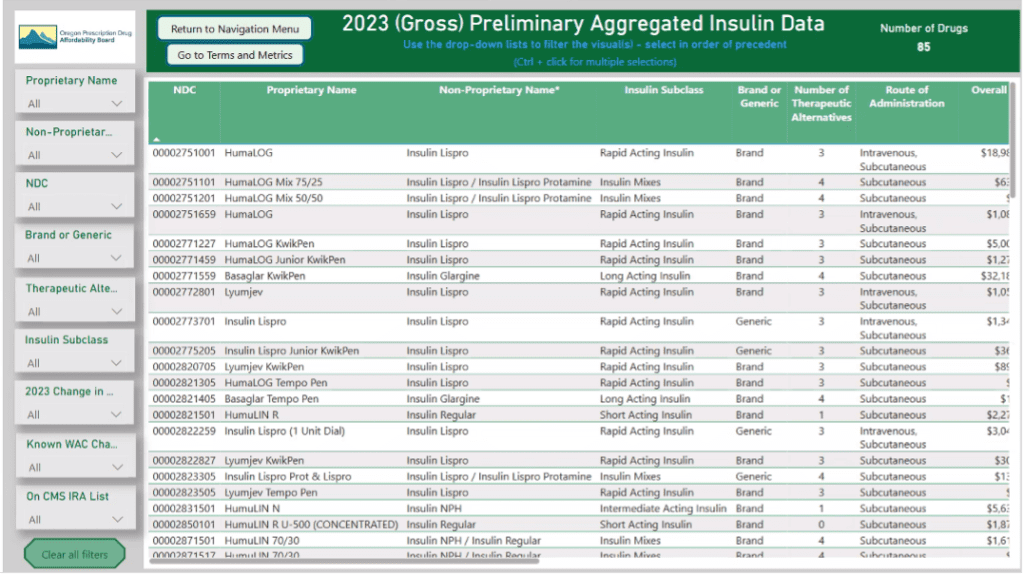
The board looked at aggregated insulin data using the data dashboard.
The Board discussed criteria for review and how to create a subset list, focusing on the following categories for analysis:
- One or two from each class, i.e. four or five in total insulin subset list
- Short acting, intermediate, long acting and rapid.
- By gross overall spend
- Combining generics with branded, including biosimilars
- High patient pay per prescription, e.g. glargine
- By non-proprietary name and subclass
- By number of patients that use the drug
The board chair reminded people to speak up at the May 21 board meeting and complete the survey about drug costs.
The next PDAB meeting will be May 21, 2025, at 9 a.m. PST.