May 12, 2025: FDA will expand unannounced foreign facility inspections, it says
Major Stories
The U.S. Food and Drug Administration (FDA) announced intention to expand unannounced inspections at foreign manufacturing facilities to ensure that domestic and foreign companies receive the same level of regulatory oversight. The agency has been piloting an unannounced inspection program with facilities in India and China over the last few years.
Read June 2020 testimony before the Senate Committee on Finance to learn more about FDA’s foreign drug inspection program.

FDA headquarters (Flickr)
This week PSM published its first Prescription Drug Freight Fraud Report, which summarizes our research into March 2025 records of semaglutide, tirzepatide, antibiotics, and apixaban shipments sent from unregistered sources. FDA import records showed shipments from very unexpected places; we offer suggestions for how to better protect American patients.
Patient safety issues in the GLP-1 space this week
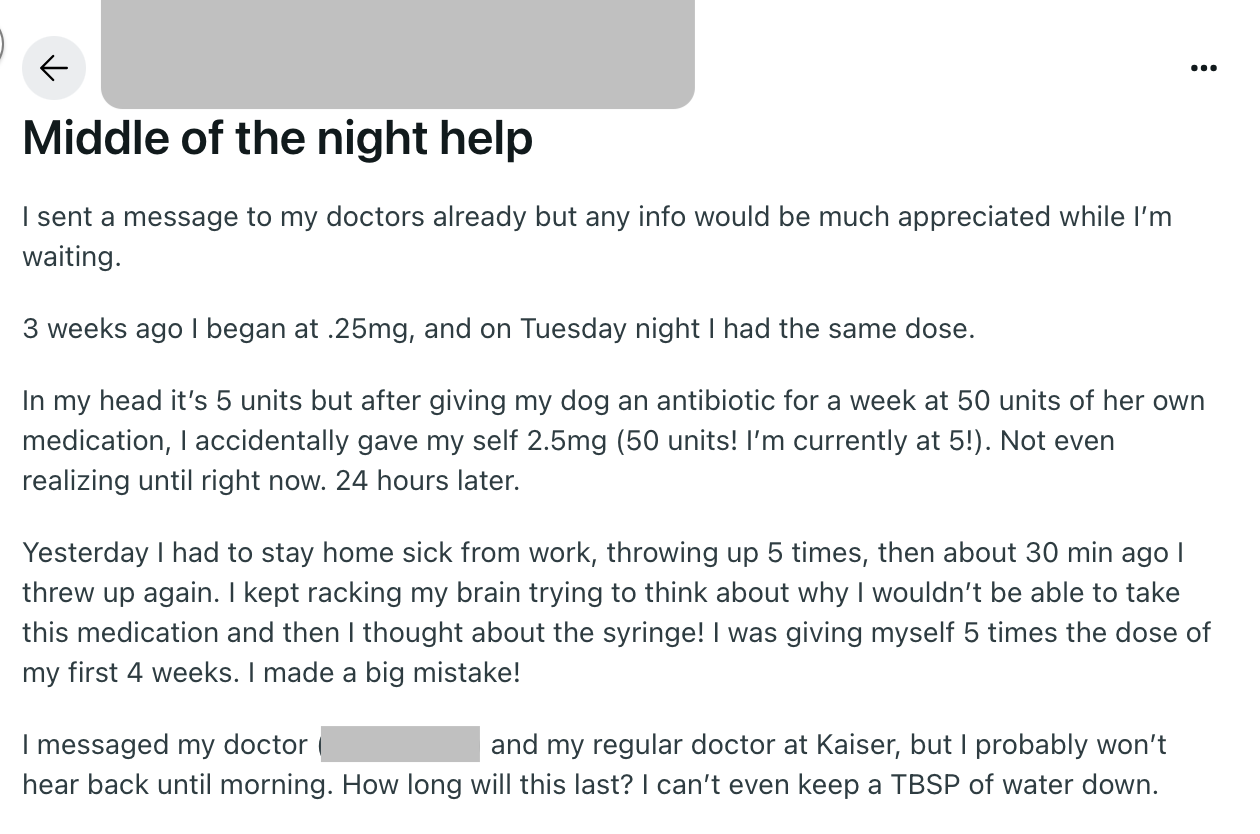
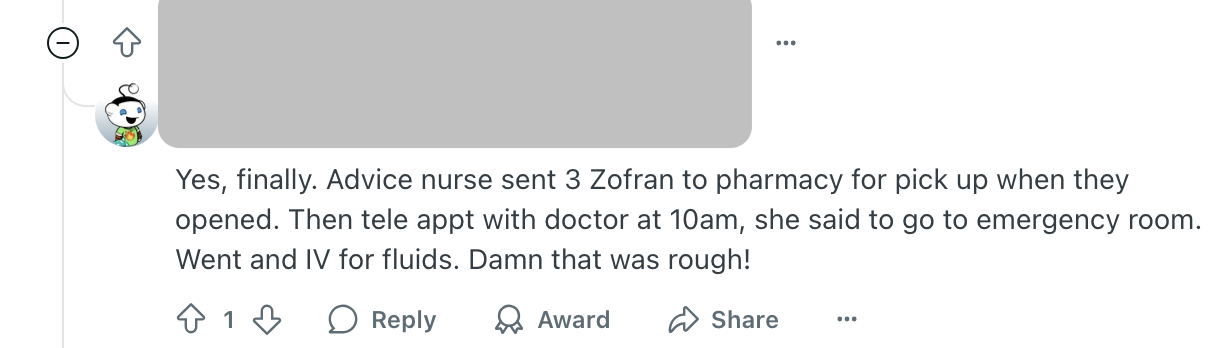
Relevant Reddit groups are riddled with posts from patients about calculating doses before injecting them and dosing errors when they’ve made mistakes. This one stands out because the patient had to go to the emergency room—a sign of the seriousness of these errors.
Domestic News
The FDA warns again about tianeptine. A Florida physician injected patients with silicone. News involving pIll presses in Massachusetts and Ohio.
The FDA reiterated its November 2023 alert warning consumers not to take tianeptine supplements because of serious risks. The unapproved supplement, which has been called “gas station heroin,” is banned in 10 states.
Physician Nhan Pham, of Orlando, Florida, pleaded guilty to receiving an adulterated medical device in interstate commerce. Between 2015 and 2019 Pham injected silicone oil into patients seeking body contouring. Although Pham told his patients that the injections were “safe” and “natural,” the victims experienced pain, inflammation, and hardness in the injection areas. In the past, people who received silicone injections have suffered disfigurement, blocked blood vessels, stroke, and death.
Last week also saw the arrest of an unlicensed aesthetician in Jacksonville, Florida. Special Agents with the Florida Department of Law Enforcement said that she had been importing and “injecting mystery mail-order facial fillers” without a medical license.
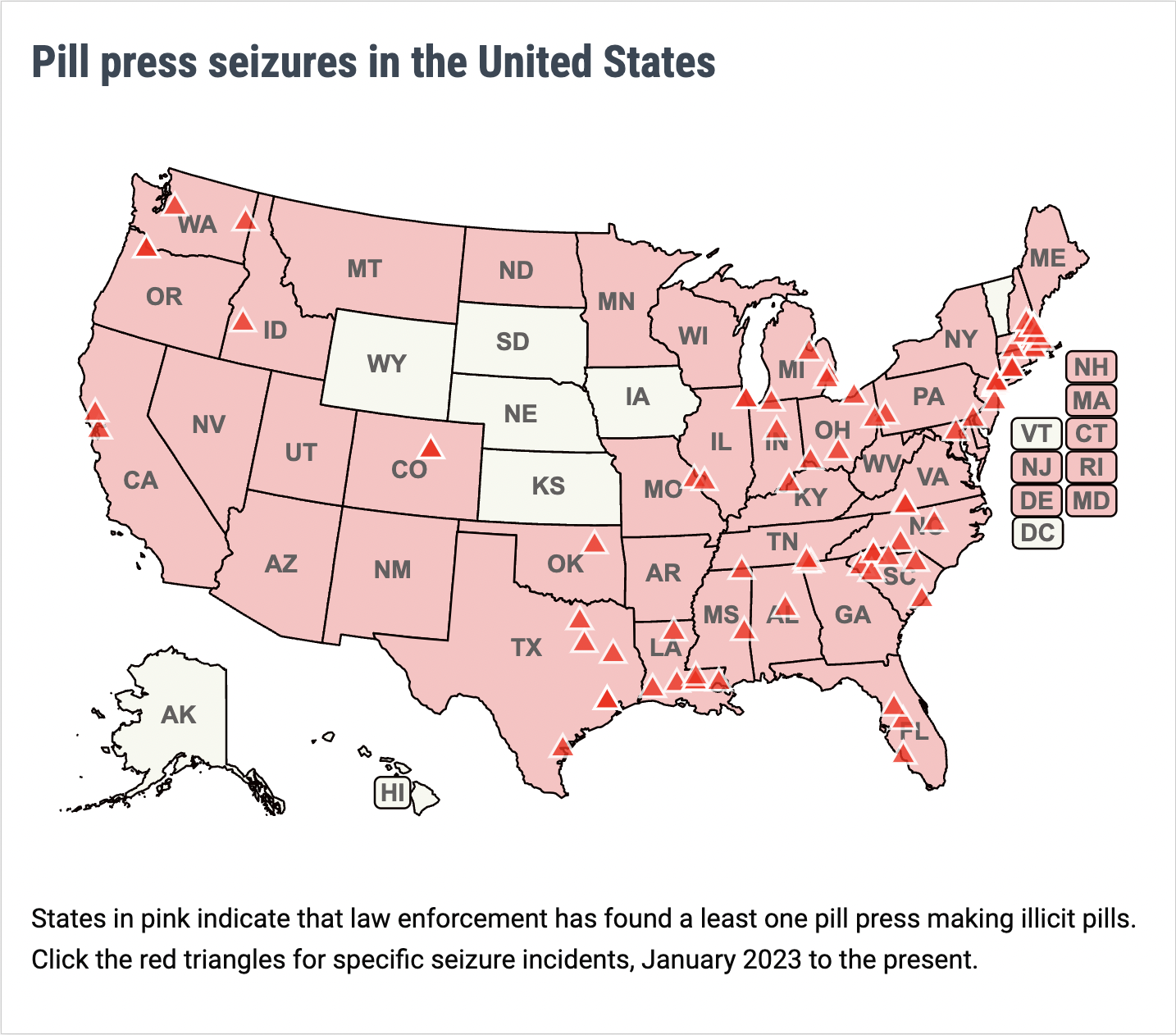
Lynn, Massachusetts resident Sebastien Bejin received a 12-year federal prison sentence for his role in a pill press operation that manufactured heart shaped candies made with fentanyl and methamphetamine. Massachusetts has been beset by drug rings like these; legislators are considering two bills which would regulate pill presses and establish criminal penalties for their illicit use.
A Euclid, Ohio resident has been charged with making methamphetamine pills and selling them as MDMA. DEA agents began investigating the man after he allegedly purchased a pill press and enough ingredients to make around 200,000 pills in 2023.
For more information about policy issues around pill presses, visit PSM's pill press page.
International News
More counterfeit Viagra and pill press seizures in Canada. Counterfeit incidents in the Philippines, India and Uzbekistan.
Health Canada announced that a variety store in Toronto had been caught selling fake Viagra pills that closely resembled the legitimate Pfizer product.
Canadian authorities seized pill presses in Alberta and Quebec.
The Philippines’ Food and Drug Administration warned the public about counterfeit paracetamol found in circulation.
Law enforcement in Delhi, India raided a wholesaler, seizing 21,000 tablets of counterfeit Vertin, a medicine that treats dizziness and vertigo caused by Meniere's disease. The drugs were falsely labelled as manufactured by Abbott India Ltd.
Uzbek authorities searched two residences in Tashkent, seizing more than 600,000 units of 51 types of illicitly-manufactured medicine; almost 170 kilograms of raw pharmaceutical materials; around 50 pieces of equipment, including machines and molds; packaging, and other supplies. Among the drugs being counterfeited were Fusys, an antifungal; Prosulpin, an antipsychotic; Ursosan, which treats liver disease; and an antiviral treatment called Arbidol.