July 28, 2025: Suspicious shipments of API continue to be sent to buyers in the U.S.
Major Stories
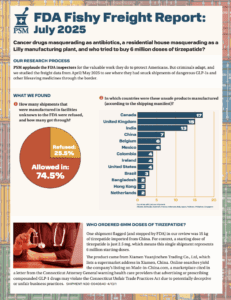
PSM released its July 2025 Fishy Freight Report. The report analyzed freight data from April and May, 2025 looking for suspect shipments of active pharmaceutical ingredients used to make medications. According to the shipping manifests, Canada, the United Kingdom, and India were the countries with the largest number of suspect shipments. While nearly three-quarters of the suspect shipments that PSM found were allowed into the country, one of the rejected shipments contained over 30 pounds of tirzepatide, the active pharmaceutical ingredient in Mounjaro and Zepbound.
PSM published this report on the same day that it was reported that Eli Lilly would no longer be partnering with Noom to sell Zepbound and Mounjaro. Noom continued to market a compounded version of tirzepatide, which Eli Lilly stated was in violation to companies’ agreement.
Domestic News
Congressmembers urge FDA to act and leader of drug trafficking ring sentenced in Florida.
A group of over 80 bipartisan members of Congress sent a letter to the U.S. Food and Drug Administration(FDA) urging the agency to do more to prevent the sale of illegally compounded GLP-1 medications from getting into the hands of U.S. citizens. The letter, spearheaded by Representatives Richard Hudson of North Carolina and Herb Conaway of New Jersey, asked the agency to work with U.S.Customs and Border Protection to stop these shipments before they can enter the country. The lawmakers have requested an update on the FDA’s efforts by Wednesday, July 30th. The full letter to FDA Commissioner Marty Makary can be found here.
Stephen Costa of Florida received a 14-year federal prison sentence after he pleaded guilty to his role as a ringleader of a drug trafficking organization that diverted and distributed $78 million worth of pharmaceutical drugs, including drugs to treat HIV and cancer, to pharmacies throughout the U.S.
This was not the defendant’s first time doing pharmaceutical crime. Costa ran shell companies that participated in a $275 million diverted HIV medicine case in New York.
Legislation
The fate of Arkansas’s House Bill 1150, which prohibits out-of-state pharmacy benefits managers from operating pharmacies within the state, remains unknown as a federal judge weighs whether or not to put an injunction in place as the case moves through the court system.
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
Patient safety issues in the GLP-1 space this week
On July 18th, a Reddit user shared that they were sent the wrong medication. Instead of receiving tirzepatide, the Reddit user received sermorelin, a synthetic form of growth hormone-releasing hormone.

Reddit post, July 18, 2025
International News
UN report on contaminated medicines, U.K. botulism cases, and increased pharmacy security in India.
The United Nations’ Office of Drug Control released an exhaustive report examining the harm done to patients around the world – primarily children – by counterfeit medicines containing diethylene glycol and ethylene glycol. The report noted that this issue has persisted for over 90 years, resulting in at least 25 reported incidents that led to more than 1,300 deaths and an unknown number of hospitalizations and injuries.
On July 18, 2025, the United Kingdom Health Security Agency released an official warning about the incidents of botulism reported in the North East, East, and East Midlands regions.
Patients in the United Kingdom continue to come forward to share their experiences after they received unapproved Botox-like injections. One woman who went to the hospital after she experienced lethargy and difficulty swallowing food reported running into other individuals experiencing the same symptoms and having to wait to be treated because the hospital had run out of anti-toxin.
After discovering counterfeit cancer drugs for sale at multiple pharmacies in June, the Delhi government in India mandated security cameras be installed in all pharmacies across the capital.