Posts Tagged ‘FDA Alert’
FDA Alert: Abbott FreeStyle Libre 3 and Libre 3 Plus Continuous Glucose Monitor Recall
The FDA has posted a notice about the recall of continuous glucose monitors from Abbott.
[...]FDA Alert: Dietary supplement recall due to undeclared drug ingredient
The FDA has posted a notice for a dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
[...]FDA Alert: Supplements with undeclared drug ingredients
The FDA has posted notices for two nationwide dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
[...]FDA Alert: Nationwide recall of Unavy and Umovy supplements for undeclared drug ingredients
The FDA has announced a voluntary nationwide recall of two dietary supplements sold exclusively online by www.umary-usa.com. Lab testing revealed that Unavy Ácido HIALURÓNICO and Umovy Ácido HIALURÓNICO contain undeclared active pharmaceutical ingredients: diclofenac, dexamethasone, and omeprazole.
[...]ALERT: New FDA Warning Letter to www.thesafepills.org
The FDA has issued a warning to the operators of the online pharmacy www.thesafepills.org for illegally selling unapproved and misbranded opioid medications, including tapentadol products marketed as Aspadol and Typendol. These unauthorized drugs, sold without a prescription, pose significant health risks such as overdose, addiction, and even death.
[...]FDA Alert: Update on Previous Nationwide Warning About Presence of Undeclared API in Capsules
The FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
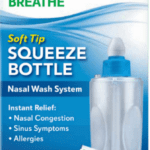
[...]FDA Alert: Recall Issued on Nasal Wash System Due to Microbial Contamination
An FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
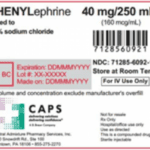
[...]FDA Alert: Recall Issued on Injectable Product Due to Potential Particulate Matter
An FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
[...]FDA Alert: Recall Issued on Dietary Supplement Containing Active Pharmaceutical Ingredient
An FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
[...]FDA Alert: Dietary Supplement Recalled for Containing Prescription Drug Ingredients
This is a reprint of an FDA Alert.
[...]