Archive for 2017
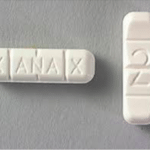
Overdoses Linked To Counterfeit Xanax Cause Police To Issue A Public Warning
Yakima, Washington Police Department issues public warning after a bad batch of counterfeit Xanax being sold on the street caused an uptick in overdoses. Police were unsure what was in the pills, but they did know they were not real Xanax and told people to stay away from them…
[...]Canadian Pleaded Guilty To Misbranded Animal Drugs Charges For a Second Time
Alain Lamontagne pleaded guilty in federal court to selling misbranded equine drugs that contained a Schedule III steroid not listed on the label. Lamontagne ran into problems with the law under very similar circumstances in 2011 in his native Canada…
[...]U.S. Postal Inspectors Are On The Front Line In the Fight To Keep Fentanyl Out Of The Country
Many government agencies are working to keep fentanyl from killing Americans. U.S. Postal Inspectors have found themselves on the front line working to keep fentanyl out of the country. The DOJ is working to bring justice to those who break our laws and the State Department is in negotiations to help stem the flow of illicit opioids into the country…
[...]Importation Will “endanger 1000s of innocent lives…in Arizona and throughout the nation,” Retired Police Commander Warns
According to retired Phoenix Police Commander Tim Hampton, who wrote this editorial in White Mountain Independent on December 15, 2017, legalizing drug importation will help “criminal organizations . . . exploit weaknesses in the law to traffic narcotics,” and increase the flow of counterfeit pills cut with fentanyl into the country: “The drug importation bill would weaken America’s anti-drug defenses and endanger thousands of innocent lives — here in Arizona and throughout the nation.”
[...]Guerra C, Mackey TK. “USA criminal and civil prosecutions associated with illicit online pharmacies: legal analysis and global implications.” Med Access @ Point Care 2017; 1(1): e104-e118.
ABSTRACT The rise of digital technologies has created a complex online environment that now includes illicit Internet pharmacies, online facilitators, advertising sites, and foreign entities. Collectively, these networks create significant patient safety risks, including acting as unregulated access points encouraging prescription drug use. Although law enforcement is active in combatting this form of cybercrime,…
[...]Illinois Resident Imported Foreign API and Pressed 80,000 Pills in Home for Sale Online
IL resident Skyler Dean Prahl pleaded guilty to introducing a misbranded drug into commerce. Prahl used a pharmaceutical powdered purchased from overseas to make Tramadol pills, which he sold online to customers around the U.S…
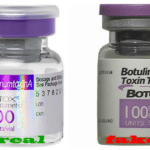
[...]Couple Sentenced For Smuggling And Injecting Clients With Fake Botox
Couple with no medical training, who repeatedly smuggled counterfeit cosmetic injectables into the U.S. and used them on their clients, received sentences for their crimes. Unfortunately, two of their clients have been left with permanent nerve damage…
[...]Pair From India Sentenced For Selling Fake Prescription Drugs to Americans
Trial initiated by an FDA-OCI investigation into a call center in India selling counterfeit and misbranded drugs to Americans ends with sentencing for the two defendants…
[...]Professor Warns That WHO Report Saying 10% Of Medicines Are Fake Is A Warning Sign
Tim Mackey, Ph.D., is an associate professor at the University of California, San Diego, School of Medicine; a fellow at the World Health Organization Collaborating Center for Governance, Accountability and Transparency in the Pharmaceutical Sector; director of Health Care Research and Policy at the University of California, San Diego, Extension; and director of the Global Health Policy Institute. In this op-ed that appeared in STAT News, he warns that the recent report by WHO that estimates that 10% of drugs in the world is fake should be a warning sign…
[...]Known Counterfeit Cancer Drug Foreign Distributor Implicated in New Case
U.S. DOJ received guilty pleas from oncologist and wife who ordered misbranded cancer drugs from QSP, a CanadaDrugs.com wholesaler, 65 times over the course of 21 months while never informing their patients…
[...]