PSM, Partners Host Fall 2019 Congressional Briefings About Counterfeit
Drug Dangers

Briefing speakers and family members of counterfeit victims in front of the U.S. Capitol.
On September 24, 2019, the Partnership for Safe Medicines and 19 partner organizations held two congressional briefings about the real dangers counterfeit drugs pose to Americans. The briefings were attended by family members of counterfeit drug victims, policy and law enforcement experts from the U.S., and Canadian health and safety representatives.
Each briefing opened with PSM board member Rick Roberts, himself a survivor of counterfeit medicine, recounting his own encounters with fake drugs and recognizing the losses of family members of counterfeit victims. Asking attending family advocates to stand, Roberts lauded each for their “bravery for sharing your story and helping us fight this epidemic of counterfeits.”
PSM board president Dr. Marv Shepherd shared the recorded remarks of Representative David Kustoff of Tennessee, who announced the introduction of the Criminalized and Abused Substance Template (CAST) Act, a bill that would increase penalties for possession of controlled counterfeit pills or possession of pill manufacturing equipment with intent to create them.
Dr. Shepherd also shared his new study, “New Pathways for U.S. Importation Threaten Canadian Prescription Drug Supply,” which examines concrete problems with exploiting Canada to lower U.S. drug prices: if 20% of U.S. prescriptions were filled using Health Canada-approved medicines, Canada’s medicine supply would run out in 182 days.
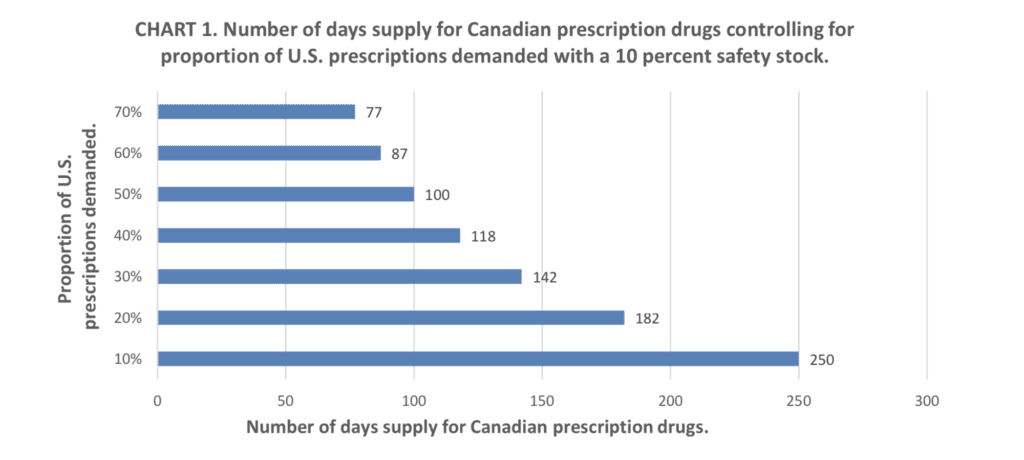
Source: "New Pathways for U.S. Importation Threaten Canadian Prescription Drug Supply," Canadian Health Policy, September 2019
Briefing Materials
Slides:
- Introduction
- Panel 1: Can You Safely Implement Canadian Importation?
- Panel 2: Cartels and Counterfeits: International Organized Crime And Counterfeit Drugs
Video:
- Entire Event (Youtube)
For copies of any of these materials, or to learn more about this issue, please contact editors@safemedicines.org.
Thanks to our briefing partners
ADAP Advocacy Association
Alabama Pharmacy Association
Alliance for Safe Online Pharmacies
Arizona Pharmacy Alliance
BeMedWise
Community Access National Network
HealthCare Institute of New Jersey
Illinois Pharmacists Association
Maryland Pharmacists Association
Minnesota Pharmacists Association
Missouri Pharmacy Association
National Association of Drug Diversion Investigators
National Consumers League
NeedyMeds
New Mexico Pharmacists Association
Ohio Pharmacists Association
Oklahoma Pharmacists Association
Rx Partnership
Texas Pharmacy Association
The first panel, “Can You Safely Implement Canadian Importation?,” which was led by PSM Executive Director Shabbir Safdar, expanded on the shortcomings of Canadian importation schemes. Track-and-trace expert Dirk Rodgers (RxTrace.com) explained why drugs from foreign markets cannot meet the safety and recordkeeping requirements of Drug Supply Chain Security Act of 2013 (DSCSA). Canada’s Best Medicines Coalition Chair, John Adams, advocated for Canadian patients who will face price hikes and even more severe drug shortages if the U.S., which has a population nine times larger than Canada’s, begins importing Canadian drugs. Adams also shared the Canadian government’s blunt response to U.S. importation schemes: “ Should any external or international actions adversely affect the supply or costs of prescription drugs for Canadians, Canada would have no choice but to respond to protect human health in Canada.” Finally, Canada Border Services Agency veteran Don Bell focused on the questionable sources of imported “Canadian” medicine, which is often transshipped through Canada without any inspection. During Operation Pangea XI, an eight-day international anti-drug counterfeiting initiative that took place in October 2018, Canadian authorities found that 87% of the more than 3500 packages they inspected contained counterfeit or unlicensed medicines. “The cost of human life, the cost to their communities and the cost to families,” Bell concluded, “far outweighs any savings you’ll get in purchasing the drugs.
Adams shared Canada's blunt response to U.S. importation schemes:
“Should any external or international actions adversely affect the supply or costs of prescription drugs for Canadians, Canada would have no choice but to respond to protect human health in Canada.”

Voices for Awareness co-founder Andrea Thomas speaking about the counterfeit drug trafficking organization that killed her daughter.
In the second panel, “Cartels and Counterfeits: International Organized Crime And Counterfeit Drugs,” Charlie Cichon, executive director of the National Association of Drug Diversion Investigators (NADDI), moderated a discussion about the scale of organized crime’s counterfeit drug operations. Andrea Thomas, who lost her daughter Ashley Romero to counterfeit oxycodone allegedly purchased from cartels in Mexico, told the audience that eleven other people had died from the same batch of counterfeit pills that killed Ashley. She urged lawmakers to institute harsher penalties that would deter traffickers:
“If you were giving your child a cancer drug that was making no difference to them and allowing them to die, or if you got a hold of a counterfeit drug that was laced with fentanyl and killed you instantly...that should have the stiffest penalty that there is.”
Armando Rivera (Bristol-Myers Squibb Security Lead and DEA veteran) showed attendees the scale of the counterfeit drug problem in the Americas by summarizing cases involving fake cancer drugs in Mexico, counterfeit rheumatoid arthritis treatments Canadian wholesaler T.C. Medical sold to U.S. medical practices, and counterfeited respiratory treatments that led to the deaths of sixteen premature babies in Colombia. Finally, retired Pinellas County Florida Sheriff’s Office captain Mark Baughman discussed “Operation Top Dog Hot Dog,” a law enforcement effort that ended in 21 arrests, and the seizure of five high-volume pill presses, almost a thousand counterfeit fentanyl-laced oxycodone and percocet pills, and more than 8,400 grams of powdered fentanyl and other illicit drugs.
In all, the briefing offered a clear picture of why importation cannot solve the problem of high drug prices in the U.S. Canada does not have enough prescription drugs to share with U.S.patients, and organized crime is poised to expand the counterfeit drug trade into the U.S. to bridge the gap without regard for the health and safety of U.S. residents. Don Bell summed up the risks to American patients when he shared a wire-tapped phone conversation between Canadian counterfeit drug traffickers: “The money to be made is far too great to worry about human life.”