Counterfeit HIV Drug Alert: Gilead Warns Of Fake Versions Of Biktarvy And Descovy Sold To U.S. Pharmacies
This is a reprint of an alert posted by Gilead Pharmaceuticals.
Gilead Warns of Counterfeit HIV Medication Being Distributed in the United States
Foster City, Calif., August 5, 2021 – Gilead Sciences has become aware of tampered and counterfeit versions of its once-daily single tablet HIV treatment regimen Biktarvy® (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and its HIV treatment and prevention medication Descovy® (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets) in circulation within U.S. drug distribution networks. Distributors not authorized by Gilead to sell Gilead-branded medicine have sold these counterfeits to pharmacies where genuine Gilead bottles have been tampered with a counterfeit foil induction seal or label and contain incorrect tablets. Working in communication with the U.S. Food and Drug Administration (FDA), Gilead has alerted potentially impacted pharmacies to investigate the potential for counterfeit or tampered Gilead medication sold by distributors not authorized by Gilead that may be within their recent supply and to remain vigilant to the potential for this to occur in the future. The authenticity and safety of Gilead-branded medicines can only be secure when obtained directly through Gilead’s authorized distributors.
Gilead continues to work closely with the FDA, pharmacies, and legal authorities to remove counterfeit and tampered medication from circulation and to prevent future distribution of these medications.
“The safety of individuals taking Gilead medication is always our first priority,” said Merdad Parsey, MD, PhD, Chief Medical Officer, Gilead Sciences. “We are taking aggressive action to ensure that healthcare providers and people who rely on our medicines can confidently distinguish authentic Gilead products from counterfeit drugs.”
Counterfeit and tampered medicines can bring serious and sometimes life-threatening health risks to individuals. These medicines are not equivalent in quality, safety, and/or efficacy to genuine medicines. They often do not contain the correct medicine or amount of active ingredient and may also contain impurities. Additionally, these medicines may be produced in unsafe manufacturing conditions and travel through insecure supply chains.
The following elements can help ensure dispensed Biktarvy and Descovy are authentic:
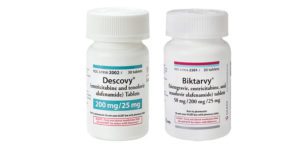
- Authentic Biktarvy tablets are purplish-brown, capsule-shaped pills with “9883” on one side and “GSI” on the other.

- Authentic Descovy tablets are blue, rectangular pills with “225” on one side and “GSI” on the other.

- FDA requires that Biktarvy and Descovy are dispensed in original packaging. Confirm your dispensed 30-count bottle of Biktarvy or Descovy are received in bottles that are white plastic, with white plastic caps, and Gilead-branded labels.
- Confirm your Biktarvy and Descovy were dispensed from a pharmacy that sources Gilead medicine directly from Gilead authorized distributors. A list of Gilead’s authorized distributors can be found here.
Individuals who believe they have been dispensed a counterfeit and/or tampered Gilead medication should immediately report the medicine to their doctor and pharmacy and Gilead Product Quality Complaints (1-800-445-3235, Option #2; QualityComplaints@gilead.com). If an individual is experiencing any side effects that may be related to a Gilead medication or to the use of a counterfeit drug, that person should immediately contact their healthcare provider and is additionally encouraged to report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch) or Gilead (1-800-445-3235, Option #3). Websites selling counterfeit and/or tampered medicines may be reported to the FDA. Healthcare professionals are encouraged to report sales solicitation of suspect drug products by emailing DrugSupplyChainIntegrity@fda.hhs.gov and by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989 or a local OCI field office.
Read the entire Gilead alert here.
Special Warning to Pharmacists
Though there are few details public because of these ongoing investigations, we believe entities with legitimate pharmacy or wholesale licenses who aren't authorized distributors have been selling counterfeits.
They likely have existing pharmacy or wholesale licenses, but lack authorization from the manufacturer.
This can be extremely frustrating because there is no central directory of authorized distributors for all pharmaceutical products we know of. Before purchasing medicine from a wholesaler who isn't your prime supplier, it is critical that you investigate whether the manufacturer has a limited supply chain list and if that new vendor is on it.
This incident of Gilead's counterfeit HIV medicines is the second time within the last year that counterfeit HIV treatments have been reported in U.S. pharmacies. In December 2020, Janssen reported that counterfeit versions of Symtuza had been discovered in U.S. pharmacies.
You can find the list of authorized distributors of Symtuza here, and the list of authorized distributors of the Gilead products here.