Prescription Drug Affordability Board Activity, June 2025
Activities Summary
Colorado: Colorado's next PDAB meeting will be held on August 22, 2025.
Maryland: Maryland's PDAB met on June 23 for updates about federal drug pricing and Maryland's biotech and life sciences industry.
Oregon: At its June 18 meeting, Oregon's PDAB reviewed survey data and narrowed its list of drugs for affordability review. The next meeting will be July 16, 2025.
Washington: Washington's next PDAB meeting is on July 15, 2025.
Activity by State
Maryland
Maryland PDAB Meeting, June 23, 2025
Agenda | Meeting Recording
CANN and Affordable Cancer Drugs Alliance submitted written comments. There were no oral comments.
Matthew J. Martin, MA, is a Program Coordinator with the Program On Regulation, Therapeutics, and Law (PORTAL) at Brigham and Women's Hospital. Dr. Benjamin Rome is general internist and health policy researcher at Harvard Medical School and a faculty member in the Division of Pharmacology and Pharmacoeconomics in the Department of Medicine at Brigham and Women’s Hospital. They presented federal drug pricing updates focused on most- favored-nation drug pricing, PBM reform, Medicare and Medicaid changes, and the 340B Drug Discount Program.
The PORTAL team presented on four executive orders relevant to the PDAB::
- E.O. 14221: Provisions related to healthcare price transparency, which include prescription drug provisions
- E. O. 14273: A slew of provisions related to drug pricing
- E. O. 14293: Promotes domestic drug manufacturing
- E. O. 14297: Implementing most favored nation pricing
Congressional:
- One Big Beautiful Bill Act, which includes drug provisions
E. O. 14297
The most-favored-nation drug pricing executive order outlined federal government actions that would align the price Americans pay for drugs with other developed nations. There are three sections to the executive order that outline how the administration imagines implementing price changes. The order
- targeted “freeloading on U.S. innovation” by directing the U.S. Commerce Department to take action against countries that artificially deflate prices paid for drugs in other countries, which would drive prices up for American patients;
- directed federal agencies, mainly HHS, to facilitate direct-to-consumer sales of drugs at the most favored nation price with manufacturers;
- instructed relevant agencies to communicate its most favored nation price with manufacturers within 30 days. This resulted in an HHS press release indicating that the MFN target would be considered the lowest price in an OECD country that has a per capita GDP at least 60% of the US’s per capita GDP. The EO also listed a slate of policy options that agencies could implement if manufacturers cannot agree on a price.
One Big Beautiful Bill Act
[Ed. note: These notes reflect the situation at the time of the meeting. PBM reform did not appear in the final pill.]
The One Big Beautiful Bill Act addressed PBM reform, specifically for Medicare and Medicaid. This section of the bill would have prohibited spread pricing in Medicaid, limit payments that PBMs give to pharmacies to the ingredient costs, and limit payments from plans for PBMs. There were also transparency provisions requiring PBMs to submit data about costs, payments, and drug fees to state Medicaid programs and HHS upon request.
Medicare provisions were included in the House bill, but not in the Senate version, which would limit payments to PBMs by Part D plans to a bona fide service fee. It would also require that the PBM-negotiated manufacturer rebates pass through to the Part D plan sponsor, and require PBMs to annually report to HHS and Part D plan sponsors about operations and the drugs and costs that are moving through the PBM on behalf of the plan. Medicaid drug reimbursement reforms were also included in this section of the bill, whic hwould require all retail pharmacies and applicable non-retail pharmacies that accept Medicaid to participate in the NADAC survey.
Medicare negotiation program
Martin also presented on the three tracks of the Medicare negotiation program currently in process. The program continues to move forward as it was designed in the Inflation Reduction Act. The 2026, 2027, and 2028 cycles are all in effect, and prices are being negotiated. The current administration commented on the 2028 cycle, prioritizing an increase in transparency of these negotiations. In tandem with these negotiations are two congressional bills that would change how the program operates. This includes the Orphan Cures Act and the Ensuring Pathways to Innovative Cures (EPIC) Act. The other policies that would affect Medicare and Medicaid include E.O. 14273, which would ensure manufacturers are paying accurate rebates and promoting innovation in Medicaid, and supporting Medicare's ability to obtain better value for drugs.
Lastly, PORTAL presented on changes to the FDA, talking about efforts to streamline both the process for drug importation through Section 804 and the regulations and guidance from the FDA and EPA for domestic drug manufacturing. The FDA has also been directed to increase fees and inspections of foreign drug manufacturing sites, and to accelerate drug approvals.
Stakeholder Council members asked about tariffs, most-favored-nations loopholes and implementation challenges, and the “pill penalty.”
Kelly Shultz, the CEO of the Maryland Tech Councils, presented on the Maryland Biotech Sector, beginning by noting that Maryland is the third largest biopharma region in the country, and one of the most sought-after for professionals. She highlighted new facilities coming to the state, including those from AstraZeneca and the University of Maryland. Shultz identified lack of growth, global competition, and policy challenges as the challenges facing the Maryland Biotech Sector. Lastly, she stressed an investment in talent for the future of the biotech industry in Maryland.
The Stakeholder Council asked questions about transparency concerns, policy priorities, and policies to reconcile the challenges China presents to the industry.
Two new members of the Stakeholder Council were introduced. Pallavi Arora from the Maryland Hospitals Association is the new representative for hospitals. Kathleen Loughran from Elevance Health is the representative for managed care organizations.
The update on the cost review study process is that they are collecting more materials and comments related to Farxiga and Jardiance through July 3.
Oregon
Oregon PDAB Meeting, June 18, 2025
Executive Director Ralph Magrish shared that the governor signed Senate Bill 289 on May 27th, which will come into effect in January. The bill changed the requirement that the board identify nine drugs a year to review for affordability challenges. Language in the new bill says they may identify up to nine drugs a year. The bill also removed the requirement for the Department of Consumer and Business Services (DCBS) to provide the PDAB with a list of drugs each quarter, rather than 60-day price increase reporting. Finally, the bill replaced the generic drug report annual requirement with a new provision that relevant content would be incorporated in the affordability review reports themselves.
Magrish also mentioned a June 23, 2025 DCBS public hearing related to rulemaking on the licensing of Pharmacy Benefit Managers and explained that the deadline to fill an open position in the PDAB has been extended.
Policy Manager Jesse O’Brian gave legislative updates, including several bills related to 340Bs and PSAOs (Pharmacy Services Administrative Organizations) that were passed and have been signed by the governor. House Bill 2149, which relates to licensing PSAOs and House Bill 3212, which relates to tightening up requirements for PBMs could pass before the legislative session ends, but have not seen movement in a while.
Gaby Gardiner (Basic Rights Oregon) spoke about including antiviral drugs in affordability reviews and how that may affect access for HIV patients. They were concerned that disruptions in access to care may negatively affect patient outcomes and that the board did not adequately take into consideration public and private patient assistance programs that support people.
Lorren Sandt (Caring Ambassadors Program) questioned the board’s definition of affordability and the inclusion of Medicare patients in its survey data.
Tiffany Westrich-Robertson from Ensuring Access Through Collaborative Health (EACH) echoed Lorren Sandt’s concerns about data that was put together for the meeting.
Mary Anne Cooper (Regents Blue Shield Blue Cross of Oregon) spoke about the board considering whether to fill Robert Judge’s vacancy with another perspective. This concerns her because Judge was the only insurer representative on the board.
Katie Chandra (Genentech) was concerned that everyone aligned on the definition of affordability, about missing data in the dashboard and about too little time to review new data, She asked that the board delay any decision until dashboard data was validated. Finally she commented on Ocrevus and Perjeta’s presence on the subset list and asked the board to review materials submitted before making any decisions on their review.
Jessica McBride (Oregon Coalition for Affordable Prescriptions) encouraged the board to avoid delays. She opined that the orphan drug designation was being misused to receive federal benefits, and they should take this into account in their decision-making process.
Dharia McGrew (PHRMA) was concerned about the transparency of and methodology for the dashboards.
Senior Policy Analyst Cortnee Whitlock presented patient survey data and took board questions. First, Whitlock presented data about the cost of drugs as reported by survey respondents.

Drug by the number of survey responses to the patient out-of-pocket cost range. (Click image to see the details)
Robert Judge asked whether data represented on the graph could be impacted by the progress patients had made towards their deductible and whether that could skew information. Whitlock clarified that the survey question asked about the most recent monthly cost and did not address deductibles. The survey was run from late March into April.
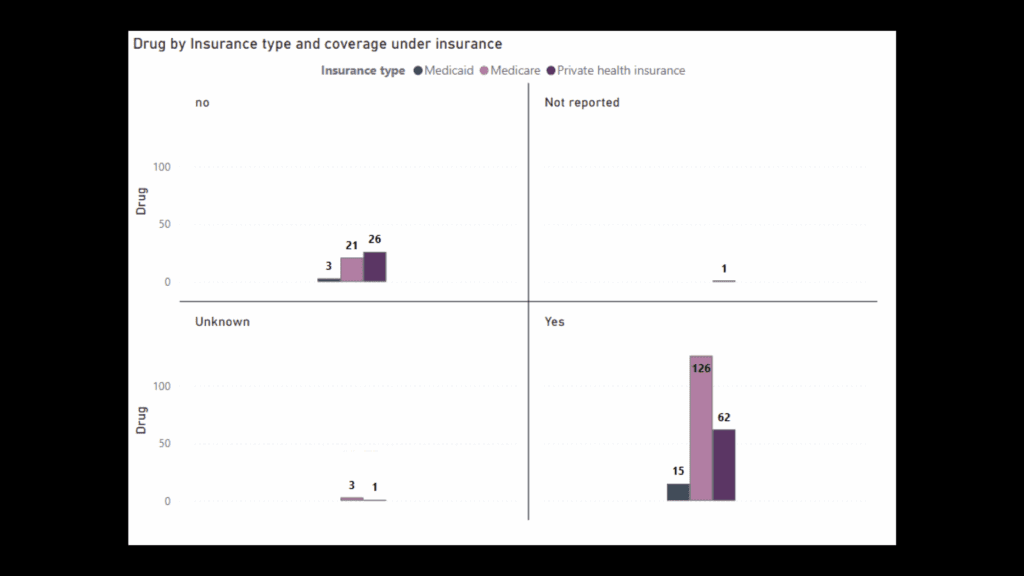
Next, Whitlock displayed graphs showing whether drugs were covered by insurance based on the type of insurance, which drugs were covered by types of insurance.
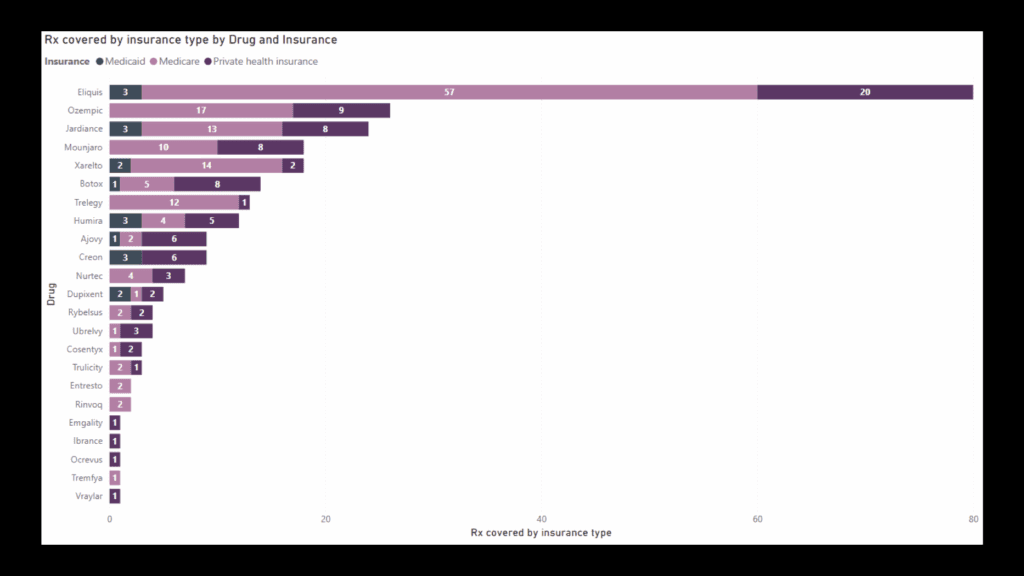
A final graph compared prescriptions covered and not covered, by drug.

Rx covered under insurance and Rx not covered under insurance based on No. of responses by drug (Click image to see the details)
The board eliminated four drugs from the subset list due to orphan designation. They spoke about which drugs to eliminate from the non-insulin subset list to narrow prospects for affordability reviews, and decided, at least for this meeting, to focus on the non-insulin products.
Chair Bailey mentioned a loose goal to walk away from the meeting with fifteen drugs on the short list.
Dr. Hartung raised concerns about a lack of definition or understanding of what affordability means.
Staff walked the board through potential filters to narrow the list. Using the DC cost, number of members, and filtered to sample size under 1,000, they narrowed the drug list to 16 and the insulin list to seven.
The list of medicines, posted here, includes Vraylar, Entresto, Ajovy, Emgality, Nurtec, Ubrelvy. Trelegy, Eliquis, Xarelto, Cosentyx, Creon, Jardiance, Mounjaro, Ozempic, Rybelsus, Trulicity, Basaglar KwikPen, Insulin Glardine-yfgn, Lantus, Lantus SolorStar, Semglee (yfgn), Toujeo Max SolorStar and Toujeo SoloStar.
Oregon's next PDAB meeting will be July 16, 2025.