August 18, 2025: PSM, ADAP ask New York’s Board of Pharmacy to act in alleged fake medicine case
Major Stories
On August 7, the Partnership for Safe Medicines and ADAP Advocacy Association filed an official complaint asking the New York State Board of Pharmacy to take regulatory action against City Plus Care Pharmacy Inc. (dba Heal The World Pharmacy). Gilead Sciences filed suit against the pharmacy in March, after it allegedly dispensed counterfeit HIV medication to a patient in Queens, New York. Read the complaint.
Domestic News
This week is National Fentanyl Prevention and Awareness Day. The VA and NIPRCC are tackling supply chain security for veterans. An Iowa pharmacy was fined, and an Indian man was denied bail in separate cases involving counterfeit Ozempic distribution. Three men were charged with allegedly distributing imported, counterfeit controlled medicines.
The Department of Veterans Affairs (VA) Office of Inspector General and the National Intellectual Property Rights Coordination Center announced “Operation Genuine Valor,” an initiative to protect the VA supply chain from counterfeit, unapproved, substandard, and noncompliant medical devices, equipment, and pharmaceuticals.
The U.S. Food and Drug Administration (FDA) warned a manufacturer in Nevada for violating Current Good Manufacturing Practice, including failing to test components used in finished over-the-counter drug products for diethylene glycol (DEG) or ethylene glycol (EG).
The Iowa Board of Pharmacy fined Washington, Iowa-based SmartScripts $25,000 for the 2023 distribution of thousands of counterfeit Ozempic pens to Central Pharmacy Management in Lansing, Michigan. The pharmacy’s license will also be under probation for five years.
A court in India denied the bail of a man who was allegedly involved in an $18.8 million scam to supply hundreds of thousands of doses of counterfeit Ozempic to a U.S. company. The FDA seized the fake injectables on their way into the country.
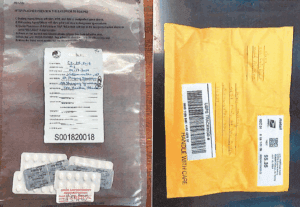
Zolpidem seized by DEA agents in Buffalo, NY, July 2024 (Criminal complaint)
Three men are facing federal charges for allegedly reshipping more than 400 packages of counterfeit controlled medicines to U.S. residents. U.S. customers ordered the drugs on fake pharmacy websites that claimed to sell medicines that originated in and were shipped from the USA, but DEA investigators traced their payments to Pakistan.
U.S. Attorneys’ offices in Indiana, South Carolina, and Ohio shared news about prosecutions involving pill presses.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
Patient safety issues in the GLP-1 space this week
On August 16, a Reddit user shared a photo of a Mounjaro counterfeit that a friend had been taking. The friend believed her medicine, which came in a box that misspelled “Monjaro,” was from a doctor’s office in Miami. The post has garnered a lot of attention, and one commenter found a matching box for sale on a Pakistan-based website. Thanks to Dave Knapp, who shared this story on LinkedIn.
August 21: National Fentanyl Prevention and Awareness Day
National Fentanyl Prevention and Awareness Day was established to remember lives lost to fentanyl poisoning and to educate the public about the crisis.
Share PSM's handout about the fake pill trade.
This year also marks a decade of Americans being harmed by fentanyl pills disguised as prescription drugs. At this sad ten-year mark, PSM urges you to talk to your family about fentanyl and take steps to protect yourself and others.




