Prescription Drug Affordability Board Activity, July 2025
Activities Summary
Colorado: Colorado's PDAB met on July 11, 2025 for a rulemaking hearing to set an upper payment limit for Enbrel, during which they heard substantial public comment. They will continue this process at their next meeting on August 22.
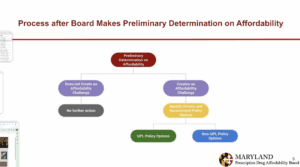
Maryland: At its July 28 meeting, Maryland's PDAB identified Farxiga and Jardiance as medicines with affordability challenges. The next PDAB meeting will be September 29, 2025.
Oregon: At its July 16 meeting, the board focused on refining the affordability review process and started reviews on Vraylar, Ajovy, Emgality, Nurtec, Ubrelvy and Entresto. The next meeting will be on August 20, 2025.
Washington: On July 15, Washington's PDAB continued to refine the data collection process for its work and chose Enbrel, Xtandi, Cabometyx, and Humira for affordability reviews. Enbrel and Xtandi will be evaluated first. The next meeting will be on November 19, 2025.
Activity by State
Colorado
Colorado PDAB Meeting, July 11, 2025
The 2024 Activities Summary Report and policy recommendations from the Board were finalized and submitted to the general assembly on July 1. There were eight nonsubstantive grammatical changes.
One PDAAC member who held a healthcare consumer position resigned, and applications are being accepted.
The PDAB has received feedback about the cost-benefit analysis in Oregon. The Oregon PDAB director was unavailable to speak at this meeting, but they are hoping to coordinate that for the next meeting.
As part of the affordability review updates, the board directed staff to start a stakeholder workgroup to identify best practices around gathering sensitive patient information. A plan has been started, and the staff is working on getting that organized with the aim of starting meetings in September.
They closed out by talking about federal policy updates and emerging trends, including the most favored nation executive order and the budget reconciliation bill. Board members requested an update from staff related to federal policy updates, including PBMs.
The board started this meeting in executive session to receive legal advice regarding the use of data by the board in setting an upper payment limit, pending litigation.
Bridget Dandaraw-Seritt (Advocates for Compassionate Therapy Now) brought up a lack of safeguards to ensure access to therapies and prevent nonmedical switching, which could result in negative patient outcomes and be expensive in the long run.
The board asked for clarification on the definition of nonmedical switching.
Dandaraw-Seritt said that nonmedical switching is something that insurance companies do to save money or make more money by switching patients to biosimilars, which could cause working therapies to stop working.
The board also asked if lowering the cost of Enbrel would cause more insurers to be able to provide the drug, increasing access and preventing nonmedical switching. Dandaraw-Seritt said that it could be great for those that Enbrel works for, but a UPL could also disincentivise Enbrel because rebates would be lower for the drug. The board raised the fact that nonmedical switching is already happening, that there's nothing the PDAB can do to legislate that, and that the UPL would include rebates.
Tiffany Westrich-Robertson (International Foundation for Autoimmune & Autoinflammatory Arthritis (AiArthritis)) raised a similar issue to Dandaraw-Seritt: patients are worried that if a UPL is set on the formulary for Enbrel that patients with the same diagnosis on a therapeutic alternative may have to pay more for their drugs.
The board reiterated that the PDAB does not have the ability to control nonmedical switching, and that the statute does allow for the board to terminate a UPL if there are unintended consequences. The board asked staff to think about how this could be done quickly, instead of needing to wait for the next PDAB meeting.
The PDAB agenda listed time for a presentation on Stelara Biosimilars from PORTAL and APCD Data Validation for the Cosentyx Validated Data Addendum and the Stelara Validated Data Addendum. Staff noted that there was insufficient time for these to take place, and suggested that the board move these items to the next meeting, which is what the board decide to do.
Rule introduction
The purpose of this rule is to establish an upper payment limit for the prescription drug Enbrel. Staff described the purpose of this hearing, which was to present data, views, and arguments concerning the draft rule to establish an upper payment limit, which will mostly include time for witness testimony.
Witness testimony
Written testimony was submitted by: International Foundation for Autoimmune & Autoinflammatory Arthritis; The Ensuring Access through Collaborative Health Coalition; Biotechnology Innovation Organization and the Colorado BioScience Association; Community Access National Network; ACTnow and Cystic Fibrosis United; Amgen, Inc; Arthritis Foundation; Children’s Hospital Colorado; Coalition of State Rheumatology Organizations; Let My Doctors DecideAction Network; Front Range PharmaLogic; Colorado Consumer Health Initiative, the Value of Care Coalition and several Colorado residents.
Tiffany Westrich-Robertson was the first to be given time to speak. She reminded the board that the figure of Enbrel costing patients $4,638 a year, which was listed in a May 2025 press release and was cited in a public comment, equaled $386.50 a month, which is often not paid because of patient assistance programs. This was reinforced in the survey run by the PDAB, where those with commercial insurance did not have an issue. She urged the board to look at what patients are saying presents an affordability problem. The board commented that they are not only focusing on insured patients; with recent federal legislation, patients will lose their Medicaid, and more patients will be uninsured.
Sophia Hennessy of the Colorado Consumer Health Initiative testified to express the organization's support for the UPL on Enbrel. She characterized the PDAB as a collaborative body with members from across the drug supply chain to establish if a drug is unaffordable, which the PDAB has for Enbrel. She cited statistics and surveys that indicated that cost affects patient access to Enbrel, and therefore, supported the board setting a UPL.
Ingrid Pan, a clinical pharmacist who works with the pediatric population with rheumatic conditions and a PDAC member, was next to testify. She urged the board and the state to carefully consider the impact of accessibility for pediatric patients when considering a UPL for Enbrel. She said that Enbrel is the preferred drug for children with juvenile idiopathic arthritis, the most common autoimmune condition affecting children. The board listed 20 therapeutic alternatives for Enbrel, but those were for the adult population. The pediatric population has 12 therapeutic alternatives, many of which require a higher burden on the healthcare system to administer. While there are assistant programs, Pan also mentioned that they may be restricted, which would force higher out-of-pocket costs. The board asked about the risk that Enbrel would be taken away from patients. Pan said that conversations with Amgen indicate that they don’t know what will happen if a UPL is set, so they don’t know if the manufacturer would take patient assistance programs, which could be a possibility. The board also asked about nonmedical switching and the ease of access for Enbrel, which Pan said was easy to access now and is a preferred product for insurers. One board member asked about the increase in price. Pan said she has not seen it in copay amounts, so it has not been a problem in her practice.
The next speaker was Melanie Kesner from the Rocky Mountain Region Director at Young Invincibles, testifying for their support of a UPL for Enbrel. She reiterated that Enbrel’s high cost, especially for young adults, is prohibitive for many, especially as many young people may lose coverage due to federal legislation. A board member reiterated the point that patient assistant programs are voluntary, and they may be taken away, or some may not qualify for them.
Bridget Dandaraw-Seritt from Advocates for Compassionate Therapy Now reiterated the point that the appeal process is not developed yet, which was something the board was supposed to do. She also mentioned that unless the UPL limits the price of Enbrel to $50 or less, Enbrel will still not be affordable without patient assistance programs.
The next speaker, Kristy Kibler of Lupus Colorado, raised concerns about the lack of guardrails in the UPL process. She asked the board to ensure there was a robust system for access monitoring in place before UPL enforcement begins. This would include benchmarks, transparent and real-time tracking of patient outcomes, insurance coverage, and provider availability. She especially stressed establishing a defined process for pausing or pulling back the UPL if there are access issues.
Katelin Lucariello, representing PHRMA, was concerned that the board had not established clear standards for weighing each category of the data you’re considering in UPL rulemaking. She also mentioned that the timeline to submit data to the data submission guide was rushed and inconsistent with what was offered during the affordability review. She would also like public comment to be before and after board deliberation, rather than just before.
Bethany Pray of the Colorado Center on Law and Policy, expressed concerns about system costs and costs to individuals. The first point she made is that Amgen charges Coloradans over $90,000 a year for a drug that was developed with taxpayer money through the NIH. She posited that the price Coloradans paid for Enbrel did not reflect their contribution to its development. She mentioned that the concerns that other organizations and advocates have regarding access issues in the event that a UPL is set implicitly give Amgen the okay to set or raise prices with impunity. Last, she shared a colleague's story of receiving $12,000 in 2022 to cover the out-of-pocket cost for Enbrel, which dropped to $7,500 in 2023 without notice. After assistance was exhausted, they had to pay $2,000 in one month for Enbrel. Pray said that it’s time for a UPL.
Amy Goodman with the Colorado BioScience Association expressed continuing concerns about UPLs as a tool to accomplish the PDAB's goals, and about the lack of a concrete methodology for determining UPLs in a clear, consistent manner. She reiterated concerns about unintended consequences and the negative effects on patients of setting a UPL, including limiting patient access to medicines, and a lack of understanding about how supply chain members will respond.
Derek Flowers from the Value of Care Coalition was the next witness. He said that no discussion of prescription drug affordability is complete without a consideration of value, which is best conveyed by patients and clinicians. Because of this, the organization commissioned a white paper surveying specialists' views on PDABs and UPLs in four states, including Colorado, where boards are operational. The survey found that specialists are concerned that PDABs have not sought their input, that they will limit available treatment options and provider autonomy, that the administrative burden will be increased, and that nonmedical switching would be increased. The board replied that they have reached out to providers and that providers are included on the board.
Emily Zadvorny, a pharmacist and representative of the Colorado Pharmacist Society, was the last witness. She encouraged the board to look at other ways to address the affordability of drugs, rather than only looking at a UPL as the only way to address affordability. The board mentioned that they are only allowed to make a UPL per their statute, though they can recommend other options to the legislature.
The board passed a motion that the UPL Rulemaking Hearing will continue to the next meeting on August 22, 2025, where they will hear more witness testimony.
Maryland
Maryland PDAB Meeting, July 28, 2025
Presentations:
Derek Flowers, on behalf of The Value of Care Coalition, presented concerns from specialists in states with PDABs, including Maryland, as reported by the organization's white paper. He said that specialists are concerned that PDABs haven’t sought their input, which puts health outcomes at risk. Specialists are also concerned that PDAB decisions would increase administrative work and reduce time spent with patients.
Harry Gewanter, a pediatrician and board member for the Let My Doctors Decide Action Network, shared Derek's concerns about a lack of frontline stakeholders. He also urged the board to recognize that therapeutic alternatives are not therapeutic equivalents.
George Huntley, a patient living with type 1 diabetes and the CEO of the Diabetes Patient Advocacy Coalition, was concerned about access to diabetes medications. He asked the board to consider UPL alternatives, like encouraging PBM reform.
Staff presented on the cost review study process. The two drugs that have been selected for the cost review study process are Farxiga and Jardiance. They are determining if these drugs have led or will lead to an affordability challenge for the state health care system or high out-of-pocket costs for patients as part of the preliminary determinations on affordability decisions.
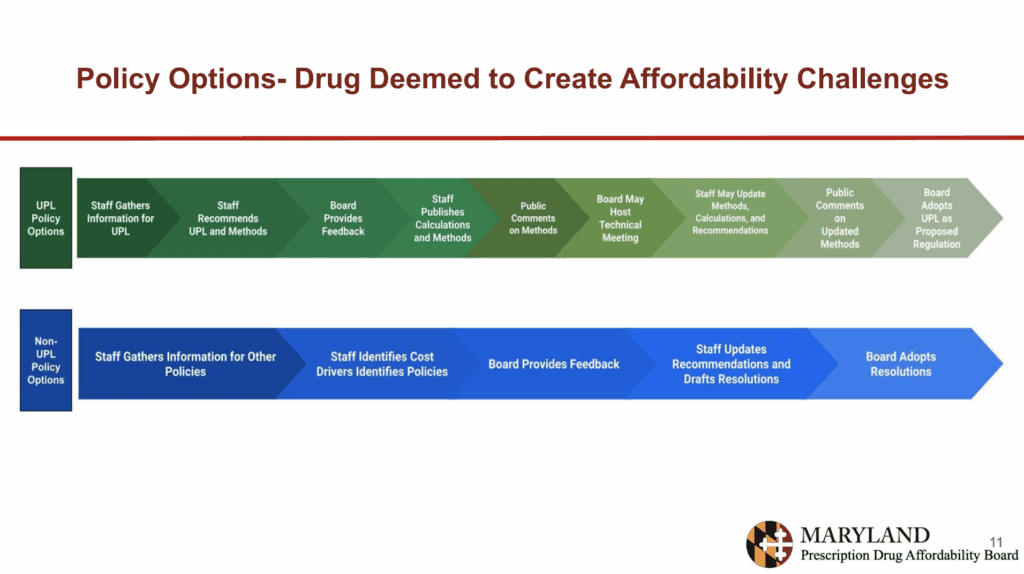
They also presented the policy options for the board as seen in this chart:
The staff presented an overview and updates on the dossier for Farxiga, which included general formatting and copy editing, updated government website links, and updated information on authorized generics. The dossier has sections about background, clinical information, regulatory approval and market context, utilization, pricing information and rebates, cost-sharing, therapeutic alternatives, cost comparisons, and health economics outcomes.
In the background section, there is information about the general background of Farxiga, including all NDC’s associated with the drug, not necessarily just those that are relevant. The clinical information section presents information about indications and a comparison to the clinical guidelines set by the FDA. It also lists some of the other drugs that could be used for these indications. In the regulatory approval and market context section, there is information about Farxiga’s FDA approval and the timeline of these approvals for each indication. It also mentions that the drug is not in shortage and when patents expire to make way for generic drugs.
The utilization section shows the total gross spending and the change in spending for the drug across markets. This section also presents information about the impact of utilization and spending for the drug on public budgets. The pricing information and rebates section has estimates of the manufacturer's net price and net sales amounts, and average price concessions the manufacturer is expected to provide to each PBM operating in the state. It also has information about factors that influence the price of the drug.
Section 6 shows information about therapeutic alternatives, their utilization, costs, and out-of-pocket costs. It also shows that there are no generics or therapeutic equivalents under other applications to Farxiga. Section 7, about cost-sharing and insurance benefit design, shows the estimated impact of patient access resulting from the cost of the drug relative to insurance benefit design, the expected dollar value of patient access programs, and the average patient copay. It also includes a literature review of the impact of the cost of the drug on priority demographics. Finally, it includes input from the public.
The board then entered a closed session to discuss confidential information concerning Farxiga and to receive legal advice. The board made a preliminary determination that Faxiga has created an affordability challenge, identifying two circumstances. In public sessions, they discussed some of the conversations that led to this determination. This included the finding that from launch to present, the WAC price increased substantially faster than overall inflation, which increased the cost to the healthcare system. The other circumstance the board found that presented an affordability challenge was the high out-of-pocket cost to the payer relative to the net price.
They also discussed that the state and local employees' percentage of total spending for Farxiga is greater than 1%. If out-of-pocket spend is a cost barrier, the above-average diabetes rates in Maryland justify the time the board is spending on Farxiga. They passed the formal resolution to find that Farxiga has led to an affordability challenge.
The stakeholder council meeting will be on August 25 and the next scheduled board meeting is on September 15. Staff is working on around six dossiers that they hope to get out for public comment before the next board meeting. Staff is looking to hire for two positions, but they are subject to a state hiring freeze and are waiting on exemptions. They came in under budget by $300,000, and some of that will carry over into this year.
Jardiance was given a similar presentation to Farxiga, with the same sections of information included. They highlighted information about cardiovascular comorbidities for patients with diabetes that differed from information about Farxiga. The utilization section showed data to demonstrate a higher percentage of overall gross spend across the different payers, which staff highlighted in the Jardiance cost review presentation. They also said gross spend has generally increased over time.
In the therapeutic alternatives section, they highlighted research, noting that Jardiance may be more cost-effective than other SGLT2s for reducing cardiovascular risk for patients with heart disease when compared to Invokana and Farxiga. The medical claims database research is similar to the Farxiga findings.
The board entered a closed session to discuss confidential information.
Back in public session, the board highlighted high out-of-pocket costs, depending on the payer and the NDC being used, relative to the net cost that many payers are paying as part of the affordability challenge. They also mentioned confidential data about the WAC increasing over time substantially faster than the rate of inflation as a contributing factor to the preliminary determination that Jardiance presents an affordability challenge.
The board formally passed a resolution that Jardiance does cause an affordability challenge.
The September 15, 2025 Prescription Drug Affordability Board meeting has been rescheduled to a virtual meeting on September 29, 2025 at 2:00PM.
Oregon
Oregon PDAB Meeting, July 16, 2025
A staff member informed the board that Executive Director Ralph Magrish has taken a different position, and they are currently seeking his replacement. The posting for the new position closed, and they are in the process of reviewing candidates.
Written comment was provided by the Let My Doctors Decide Action Network, Sanofi, Arthritis Foundation, the Pharmaceutical Care Management Association, the Oregon State Pharmacy Association, Novartis, Pfizer, Oregon Coalition for Affordable Prescriptions, e Community Access National Network, Bristol Myers Squibb, Ensuring Access through Collaborative Health and the Patient Inclusion Council, Caring Ambassadors, and several Oregon residents.
Ranier Simons from the Community Access National Network (CANN) thanked the board for officially removing Odefsey from the list of medications selected for affordability review and for acknowledging the impact of patient access programs. He still expressed concern about the number of reviews planned per meeting.
Lorren Sandt from the Caring Ambassadors Program raised concerns about how affordability is defined and about the plan to review six drugs in one meeting. She also said there is not enough patient and provider feedback.
Anne Murray from Bristol Myers Squibb urged the board to remove Eliquis from the list for affordability review. She cited the fact that Eliquis had already been selected for and completed the process for the Medicare drug price negotiation program, and the maximum fair price will begin on January 1, 2026. She also said that studies, including the board's own surveys, show that Eliquis is affordable for Oregonians and that the drug will soon face generic competition.
Primo Castro from the Biotechnology Innovation Organization was also concerned about the lack of definition for affordability and a standardization of the review process, and the number of affordability reviews in a single meeting. He also mentioned that the patient community was concerned by a board member's comments that they should consider recommending to the legislature that the orphan drug exemption be removed from the Oregon PDABs review process.
Vanessa Lathan from Ensuring Access through Collaborative Health (EACH) and the Patient Inclusion Council (PIC), urged the board to continue to narrow the number of drugs eligible for review and to keep in mind the risk of creating access challenges by saying that a drug is unaffordable.
Dharia McGrew from PhRMA highlighted math errors that cannot be explained in every report, including in the review summary, as well as discrepancies in the updated gross vs net price in some reports.
The senior policy analyst presented information about the step of the process the board is in. She mentioned that the board identified 16 drugs for review, which staff has split into four “buckets” of drugs.
The first bucket consists of the antipsychotic Vraylar, the cardiovascular agent Entresto, and the four migraine products: Ajovy, Emgality, Nurtec, and Ubrelvy, which are being reviewed in this meeting. She asked which group the board wants to continue with in future meetings. The board had no opinions about the order, but may prefer for the glargines to be last. One board member echoed public concerns about the volume of drugs in each review, and suggested narrowing down the most useful data the review packets for the board.
Staff then turned to the concerns about the information in the packet and how that can be improved upon. One board member summarized his concerns, including the structure of the reports being scattered because of the amount of information staff is trying to include in the packets. Staff clarified that the volume of information is required by statute, though there were edits to the structure suggested.
The board began presenting drug information about Vraylar, largely talking about the structure of the packets and what they would like for staff to change in the future.
Board Chair Shelley Bailey asked staff to remind the board of the three metrics that the board decided to look at to help establish the second round of the subset list: data call information filtered by largest to smallest cost; the number of enrollees by claim line of business; and the removal of drugs that had less than 1,000 enrollees. The Chair suggested that they use this as a starting point for an affordability review. The board did not come to a conclusion about the affordability of Vraylar and decided to turn to the migraine product review before drug-specific public comment.
Initially the board chair asked to talk about the total spend per claim as a statistic that stood out with these drugs. A board member mentioned that they were struck by how few minority groups are treated for migraines, and noted Nurtec and oral medications have a slightly broader possible use from a clinical perspective.
The board also discussed the frequency of use for each of the drugs, some of the cost data for the drug options based on the dashboard data, and survey data. They did not come to any specific conclusions.
David Gross, from Pfizer, commented on Nurtec. He wanted to make the board aware of the recently published statement from the American Headache Society on the use of CGRP targeting therapies for the prevention of migraine that concludes these therapies, such as Nurtec, should be considered as a first line therapy without prior failure of other classes of non-specific preventative medications. He also noted that Nurtec is not on the preferred list for Medicaid. A board member asked if this drug is used for both prevention and acute pain, and he clarified that Nurtec is used for both.
Lorren Sandt, from the Caring Ambassadors program, spoke about Vraylar, encouraging the board to be mindful of the number of Medicaid patients that are on Vraylar.
There was a short, high-level discussion about Entesto. Staff highlighted the lack of a therapeutic alternative, and talked about APAC spend, total spend, and the number of enrollees. A board member commented on the price concession for the drug being significantly less than other drugs, which could reflect the competitive environment for the drug. Staff also noted that the average out-of-pocket cost per enrollee is very different in the small group vs the large group.
The next board meeting will be on August 20, 2025, and will start an hour earlier than usual, at 8 a.m. Pacific.
Washington
Washington PDAB Meeting, July 15, 2025
The director reported that the fifth board member position was still open, though a new HCA director had been found and would start on August 18th. In terms of budget, things have calmed down since the spring. There was a small decrease in the budget, though the director did not seem to be concerned. He mentioned that the Colorado lawsuit was dismissed, and there were no updates about that. In response to a public comment, the director clarified that physician-administered drugs were not exempt from the price list because some are dispensed at specialty pharmacies.
Staff presented a few updates to the dashboard, including the ability to view both specialty and non-specialty drugs in one tab, a terminology change from specialty or non-specialty to biologics and non-biologics, and the ability to visualize how biologics and non-biologics compare.
The board decided that there will be a handful of drugs (from 3-6) to put on the list and then pick some to prioritize for affordability reviews. The board decided that the top four drugs in the list of out-of-pocket costs for both biologics and non-biologics should be the drugs selected for affordability review, focusing on the top two first. This means that Enbrel, Xtandi, Cabometyx, and Humira were selected, and Enbrel and Xtandi will be evaluated first.
The board discussed the fact that Humira has therapeutic alternatives and whether that should disqualify it from review. They decided that they would still review it, but prioritize it last. The board passed a formal motion to review Enbrel, Xtandi, Cabometyx, and Humira, in that order.
There were significant updates to the manufacturer information submission form for affordability review, based on board suggestions. Those included clearer instructions and the removal of sections for pricing for foreign countries and for advertising and marketing costs. The staff asked the board if they require the CMS pricing questions to get historical data. The board said they may need that data, so the questions should be required.
The staff also wanted to discuss patient and medical expert surveys. In the patient survey, the board discussed whether the survey should be open to Washington residents, who will be targeted to fill out the survey. They also considered the length of the survey. For the expert survey, the board discussed the wording of questions and whether doctors would be able to fill out information that is usually dealt with by medical administrators. The main feedback for both surveys was to shorten them.
Bristol Myers Squibb, Community Access National Network, Ensuring Access through Collaborative Health (EACH) and Patient Inclusion Council (PIC) and Gilead Sciences submitted written comment.
Dharia McGrew, on behalf of PhRMA, asked that the manufacturer data call template be posted to the website before it’s finalized, and that the patient and provider surveys be posted to the website. She mentioned that one problem with another PDAB was that patient advocate groups were not included in the process.
Seth Greiner, with the National MS Society, seconded Dharia’s remarks about including patient advocacy groups in the surveys.
Vanessa Lathan from Ensuring Access through Collaborative Health (EACH) and the Patient Inclusion Council (PIC) urged the board to include patient voices and patient advocacy groups in the decision-making process.
The next meeting will be November 19, 2025.