Prescription Drug Affordability Board Activity, August 2025
Activities Summary
Colorado: Colorado's PDAB met on August 22, 2025 for to continue rulemaking to set an upper payment limit for Enbrel, during which they heard substantial public comment. They will continue this process at their next meeting on October 3.
Maryland: The next PDAB meeting will be September 29, 2025.
Oregon: At its August 20 meeting, the board reviewed drug review methodology and began discussions about Trelogy, Eliquis, Xarelto, Cosentyx, and Creon. The next meeting will be held on September 17, 2025.
Washington: On August 12, Washington's PDAB Advisory Group discussed its role in the next steps of the affordability review process . The next PDAB meeting will be on November 19, 2025.
Activity by State
Colorado
Colorado PDAB Meeting, August 22, 2025
Board Member & Director Updates
Director updates included a note about skipping general public comment to make time for the rulemaking hearing, which staff clarified was not a new normal. Staff asked that written comments be sent to the board's email and noted that they anticipate an additional hearing on the Enbrel UPL. They also acknowledge comments about the flow of the hearing and the fact that the comment is at the end of the meeting. Lastly, staff said there were still open PDAAC positions.
Rulemaking Hearing - Staff Data Presentation
Staff clarified that the purpose of this rulemaking hearing was to establish an upper payment limit (UPL) for Enbrel. The board determined that the drug is unaffordable for Colorado consumers during drug reviews.
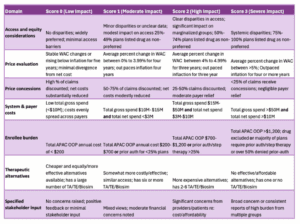
Staff presented information to the board, starting with a recap of how the board arrived at this point in the rulemaking process. Staff also added three new metrics for consideration by the board in rulemaking hearings: drug shortage information, retail coupon amounts, and impacts to older adults and people with disabilities. They did not find a shortage for Enbrel or its therapeutic alternatives since monitoring started, and their retail coupon analysis showed the cost of the coupon amount ranged from $5 to $12 on GoodRx. Staff also updated the graph packet with additional information about the age distribution of Enbrel users in Colorado. They mentioned that they will not consider research and methods that employ dollars per QALY or similar methods and that they've reached out to many stakeholders to encourage testimony.
The board requested a review of some of the graphs staff put together. While looking through this, the board discussed how a UPL would be enforced and how pharmacy savings would be passed to consumers, which the statute requires transparency around. They also spoke briefly about the Medicare Maximum Fair Price (MFP) and how it could affect drug prices.
The board entered an executive session to talk about confidential pricing information.
Rulemaking Hearing - Board Deliberation
The board discussed using the MFP to arrive at an upper payment limit number or range. One member supported using the MFP to leverage research and negotiations the government had already done. Another member agreed that they should use the MFP: pharmacies will be offered drugs at that price, and there are already mechanisms in place.
The board reviewed who the UPL would apply to and discussed how the UPL would affect pharmacy economics. Members discussed the idea of setting the UPL at a percentage over the MFP, including one board member, who proposed setting the limit at 5% over the most recent MFP, or another number that allows for inflation. They also discussed what would happen if Medicare stops negotiating MFPs.
The board entered an executive session to receive legal advice on whether it could reconvene to reset the UPL criteria if the MFP should go away, as well as what the methodology would be if a drug does not have an MFP. Members concluded they could set a UPL based on the best data available in the absence of an MFP.
The board amended and approved the draft rule, adding a reference to the dollar amount of the MFP and specifying that that figure must be revisited annually.
Rulemaking Hearing - Witness Testimony
12 people spoke at the meeting. Additionally, written testimony is here.
Vanessa Lathan, with the Patient Inclusion Council, raised concerns about the impact of the UPL process on patients, asserting that
- any UPL the board sets will still be unaffordable to vulnerable uninsured patients.
- UPLs do not address the systemic drivers patients have identified, and
- there has not been meaningful engagement from impacted communities.
The board chair acknowledged that UPLs will not make drugs affordable to uninsured patients, but that UPLs are the only tool the board has the power to implement.
Tiffany Westrich-Robertson, with Ensuring Access through Collaborative Health and the Patient Inclusion Council, suggested that the board had not looked into the “why” behind patient-reported affordability challenges. Patient Inclusion Council survey found that changes in insurance out-of-pocket costs, accumulative costs, and insurance delays were some of the factors creating affordability problems and causing patients to skip or stretch doses.
The board asked Westrich-Robertson to expand on the survey findings with non-Medicare patients. She responded that regardless of the drug, self-reported affordability challenges varied because of insurance and a lack of education about patient assistance programs.
Katelin Lucariello, representing PhRMA, was concerned that the board was moving forward with a UPL without providing clear guidance on how it would be implemented and without establishing a process to review and rescind it. She said these processes could affect insurance coverage, which could impact pharmacy reimbursements and patient access downstream, and brought up operational and legal issues around using the MFP.
The board chair responded that staff was working on a process to review UPLs at any point after they are set.
Primo Castro, on behalf of the Biotechnology Innovation Organization, was concerned with the lack of transparent and consistent methodology in the rulemaking approach when setting the UPL. He said UPLs will disproportionately impact vulnerable populations by encouraging formulary exclusions, placement on less favorable tiers, and stricter utilization management.
The board asked for clarification about how a UPL would cause a drug to be excluded from formularies or put on a higher tier. Castro replied that not all members of the ecosystem had been questioned.
Bethany Pray of the Colorado Center on Law and Policy supported using the MFP to set the UPL price. She said that patient assistance programs are often helpful for patients, but providers have told her that it’s difficult to enroll patients in those programs. She also said that a UPL would help with premium increases in the future. Lastly, she expressed the hope that the PDAB’s actions would guide pharmaceutical companies in avoiding regulation.
A board member asked Pray how payers would react to a UPL. She thought that other manufacturers would look at the UPL set on Enbrel and proactively lower prices to avoid having UPL sets on their drugs.
Amy Goodman from the Colorado BioScience Association testified that the PDAB had no clear or consistent methodology for determining UPLs, since there had been no discussion of how to set a UPL with no MFP or for updating UPLs. She was also concerned that the board had not sufficiently explored to whom UPLs would apply, how they would be implemented, or what effects they would have down the supply chain. She referred to survey results published by Avalere that supported the idea that payers and PBMs made changes to formulary changes largely based on changes in rebates.
The board responded that, given the complexity of the system, they look at all available data when considering a UPL.
Derek Flowers from the Value of Care Coalition followed up on a conversation that ensued from the last meeting related to his testimony. The board said they’d like feedback from insurers and PBMs about their plans to manage the impact of UPLs once they’re set, but there hadn’t been a follow-up. Citing the Avalere survey mentioned in previous testimony, he flagged potential pharmacy stocking issues, formulary changes, and patient access issues.
The board chair again asked about the why behind these assertions. Flowers replied that asking relevant stakeholders would be the best way to get a response and that there is more information in the Avalere survey.
Austin Blumenfeld of Centennial State Prosperity testified in support of a UPL on Enbrel. He explained how often his organization hears about the cost of prescription drugs in their townhalls and surveys. He said that Coloradans support the board and that many people are overwhelmed by the cost of prescription drugs.
Candace DeMatteis from the Partnership to Fight Chronic Disease talked about the Avalere study, which they commissioned, to ask insurers about the potential impact of UPLs, which found that patient costs would not decrease, but patient access likely would.
One board member asked how a UPL would cause higher prices or disruptions to pharmacies.
DeMatteis replied that the UPL would be set on the pharmacy reimbursement, not the acquisition cost.
The board and staff clarified that this was incorrect; the UPL would be set on how much the pharmacy pays for a drug.
DeMatteis mentioned that insurers also identified the implementation and renegotiation of some multi-year contracts as an area of costs.
Bridget Dandaraw-Serritt (Advocates for Compassionate Therapy Now) seconded Vanessa Lathan's comments, adding that she was concerned about formulary changes based on UPLs’ impacts on rebate structures, which would incentivise the use of other medications. She said that there needs to be a waiver process for patients who cannot access a drug because of a UPL standing in the way. Lastly, she opined that UPLs may encourage alternative funding programs.
The board asked how she saw the waiver process working; she replied that it should be on an individual basis for patients who cannot take any other medication. One board member expressed support, especially in the case of insurance providers requiring step therapy; other board members questioned whether waivers are in the scope of the PDAB, or if that would need to be addressed with pending PBM legislation. The board also asked her to expand on some of the problems with alternative funding programs.
Ranier Simons from the Community Access National Network was concerned about the board moving forward with deliberations without solid patient protections.
Staff responded that they were planning to present the outline of the monitoring plan—including prospective rate review filings, carrier formularies, and tiering—later in the meeting. They encouraged public feedback on this process.
Emily Zadvorny from the Colorado Pharmacists Society raised concerns about how drugs with UPLs would impact pharmacies and, therefore, patient access to drugs. She commented that this discussion was different from the Medicare drug price negotiation program, which, she said, as many as 90% of independent pharmacies were declining to participate in because there was no guarantee that they could stay afloat. She urged officials to protect a margin for pharmacies rather than leaving private companies to determine the margins in their contracts. She also encouraged the board to ensure a fair dispensing fee for pharmacies.
A board member sympathized with Zadvorny’s concerns, requested that staff pull data around the average administration fee, and asked whether there was some way to include this in the rule. Another board member was unsure if the PDAB statute acknowledges dispensing fees. The board chair asked staff to look into these numbers and see whether the statute would allow the PDAB to do anything regarding dispensing fees.
The board voted to continue the rulemaking hearing in the next meeting.
Board Business - APCD Data Validation - Cosentyx Validated Data Addendum
Staff presented affordability reviews that included the corrected data for Cosentyx, Stelara, and Enbrel for approval. Incorrect data was the result of the miscategorization of claims made by one of the PBMs that reported into the APDC. Staff determined that Cosentyx was still eligible for the process. The board looked at some of the updated data and discussed some of the statistics, including increasing copayments and overall cost, and determined that there was an affordability issue for Coloradans. They moved to approve the Costentyx affordability review summary with the addendum, confirmed that there is an affordability challenge, and to begin UPL rulemaking for Cosentyx.
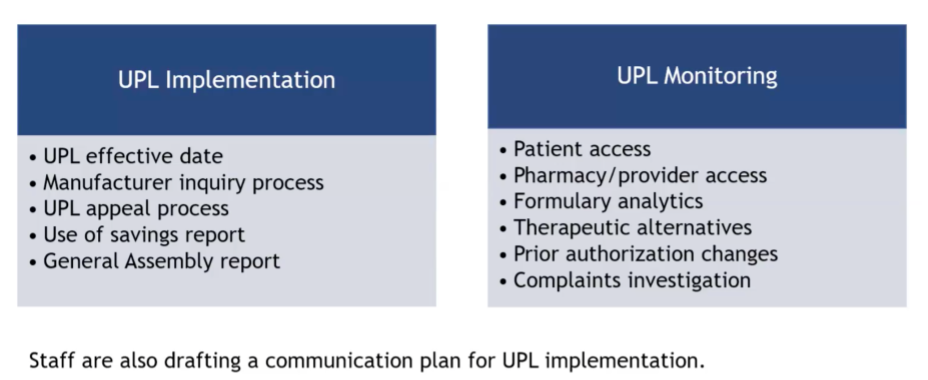
Board Business - UPL Implementation & Monitoring
Staff presented the steps involved in implementing UPLs and fielded questions from the board. Afterward, they looked at a broadly outlined monitoring process for after a UPL is in place. The staff member mentioned that they were expecting to take a year to see how indicators are trending. In response to a board member's question, staff clarified that they will examine whether prior authorizations are becoming more stringent, rather than just the quantity of them.
The PDAB ran out of time and did not get to other agenda items.
The next PDAB meeting and continuation of the Enbrel rulemaking hearing will take place on October 3, 2025.
Oregon
Oregon PDAB Meeting, August 20, 2025
Program Update
The senior policy analyst shared that the Governor's office, the legislature, and leadership at the Department of Consumer and Business Services have authorized the board to extend the drug review process into 2026, with a final report due no later than March. The board will have more time to evaluate the 16 drugs and seven insulin products currently under review. It was requested that a progress report be submitted by the end of the year, which should include an outline of the prescription drug price trends, the current drugs under review, and policy recommendations from the board. Work on that report will begin in September.
The analyst also reported that the recruitment for the PDAB and Drug Price Transparency Program executive director position had closed, and candidates were being reviewed. Lastly, at the time of the meeting, the Governor's office was still considering applicants for the vacant board member position, and staff hoped to have an update at the September meeting.
General Public Comment
Ranier Simons from Community Action National Network thanked the board for their hard work and thorough discourse concerning the nuanced aspects that affect drug costs. However, he expressed concern over the lack of a clear review process, citing the July meeting, when members spent a significant amount of time highlighting issues in the data and the board did not have a consensus about which metrics would be most important in determining if a drug is unaffordable.
Tiffany Westrich-Robertson from Ensuring Access Through Collaborative Health and Patient Inclusion Council spoke about the importance of finalizing the methodology the board will use. She also spoke about low participation rates in public surveys, raising concerns about how patient voices will be included in decision making. She said that the surveys did not ask questions that would clarify why someone can or cannot afford their medications. Given the lack of participation, she encouraged the board to read a pilot study released by her organization, which shows that drugs’ retail costs are not impacting whether or not a patient reports their medicine unaffordable.
Dharia McGrew, on behalf of PHRMA, addressed errors and inconsistencies she found in the draft scoring framework presented in the meeting materials. This included terms that were not clearly defined, quantitative cutoffs that did not have a described rationale, and general underdevelopment. She also raised concerns about the vague meanings of terms or inconsistencies in data, like net pricing being higher than gross pricing with no explanation.
Derek Flowers from The Value of Care Coalition shared concerns about the number of reviews and the PDAB’s ability to thoroughly review each drug. He was also concerned about providers not being a part of the discussion.
Johnson & Johnson representative Silas Martin asked the board to clearly define affordability, correct calculation errors that appear to mischaracterize out-of-pocket cost estimates for patients, remove irrelevant data from the dashboard (like Medicare data), and allocate sufficient time to review each drug.
Lorren Sandt from the Caring Ambassadors Program asked that the board allot time for each drug in the agenda so that stakeholders could be present for public comment for each drug. She was concerned that the scorecard lacked transparency regarding its origins and whether the board had the votes to approve its language and scoring. She also felt that there appeared to be a lack of concern about patient input in the process. She urged the board to consider nuances in the conversation about therapeutic alternatives and stakeholder impact.
Jessica McBride from the Oregon Coalition for Affordable Prescriptions testified that the healthcare system is failing Oregonians. She encouraged the board to continue to pursue affordability and said that patient voices are undersized in public comments.
Board Review of Methodology For Drug Reviews, Scoring Rubric, and Worksheet
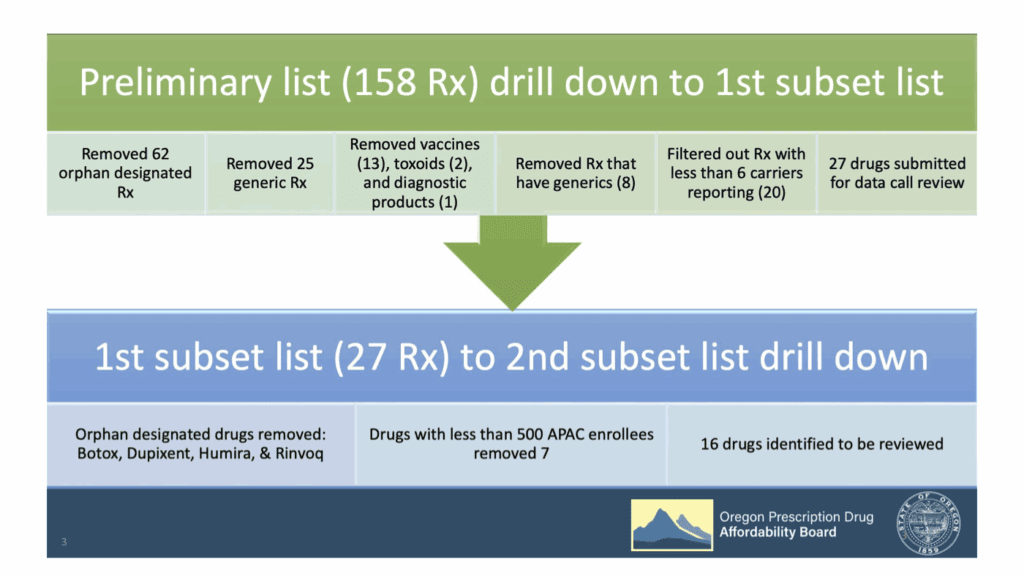
Staff revisited how the board narrowed down the list of drugs to clarify how they got to the final list of 16 drugs and the four groups being reviewed in each meeting.
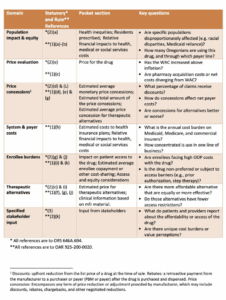
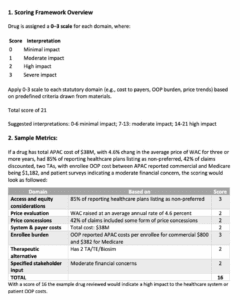
Next, they turned to the proposed scoring rubric, which is broken down into domains that follow the statutory requirement, and lists which section of the packet each domain is in. The rubric also lists key questions that the board may ask for each domain, though staff requested feedback on this in particular.
A board member said that it was helpful to clarify the thought processes, though he was unsure about the explicit scoring part. He pointed out an inconsistency in some of the domains between the two pieces of the rubric. He also said he would like to see a population impact and access domain to determine how these drugs impact the population. Another board member agreed with this idea, and would like to see administrative burden as a part of this.
The board chair suggested they discuss how the board would use this rubric. She said that the board could use the rubric as a tool, but should not use the scoring as a quantitative method for decision making, particularly towards the end of reviewing drugs. The board agreed with this sentiment.
To improve the rubric, a board member talked about price concessions, particularly the most effective metrics to be included in the rubric to reflect the impact to the payer, which could be focusing more on the overall rebate percentage. They asked staff to focus on data rather than subjective questions for now to nail down the information that should be included in the rubric. There was a conversation about therapeutic alternatives, and whether the existing information showed the full extent of other options patients have. There was a suggestion to add a domain about patent information, as well as keeping the therapeutic alternative domain. The board wrapped up the conversation after having given staff feedback.
Trelegy Drug Review
Drug specific public comment
Dr. Tom Coridge, senior medical director at GSK, commented on his experience treating patients with Asthma and COPD. He noted that Trelegy is the only inhaled drug that contains three medications, which is ideal for patients. He also talked about GSK’s patient access programs and the relatively low cost for many patients.
Discussion
To begin the discussion about Trelegy, the staff referred to the questions that are included in the rubric. One board member questioned the figure for wholesale acquisition cost (WAC) per 30-day supply, asking for clarification whether the price was per unit or per 30-day supply. They told staff that table titles and some wording was confusing. The board also asked that staff add consumer price index numbers to the table. One board member questioned whether there is a need to include the statistics and questions about average pharmacy cost in addition to the WAC, as they feel repetitive. The board decided that they are valuable and should be kept. The board also asked about the possibility to get inequality data more specific to Oregon, and staff said that that is a hope for the next iteration. Clarifying questions about various statistics continued, including those about out-of-pocket costs.
Eliquis drug review
Eliquis had no drug-specific public comment. The board began by talking about prescribers shifting away from Pradaxa, a less costly therapeutic alternative to Eliquis, in 2022 and2023. Staff was not sure what caused this, but they did make a note to research it and add it to the packet for future reference. A board member also asked for data to be broken down to better understand how much people pay for drugs.
Xarelto drug review
Public comment
Lisa Pulver from Johnson & Johnson asked the board not to find that Xarelto poses an affordability challenge because, she said, Xarelto should not have been eligible for review because it has an FDA-approved generic. She added that it only appeared on one of the three lists that the Oregon legislature requires the board to prioritize when selecting drugs, and that Xarelto will be subject to a [Medicare] maximum fair price, which goes into effect in January 2026. She mentioned that J&J patient programs offer affordable access to Xarelto, and that the PDAB’s data demonstrates the affordability of Xarelto, especially in relation to other drugs.
Discussion
The board asked whether there was a generic of Xarelto available, in response to the public comment. The board acknowledged that they were looking at data from 2023, and the generic came out in 2025. It wasn’t clear if this generic is available or just approved, but staff clarified that it was not an option while the board was selecting drugs, so Xarelto was still eligible for review. The board discussed whether a generic alternative meant that Xarelto will be deemed affordable or if the board needed to see the impact of the generic. The board learned toward relying on staff-provided data rather than weighing the impact of current or impending approvals of generics. The board also asked staff to add warfarin to the therapeutic alternative section.
Cosentyx drug review
Public comment
Tiffany Westrich-Robertson spoke about Cosentyx, saying that it was listed as a dermatology drug, but can be used for much more. She offered her experience finding a drug that worked for her, as Cosentyx did, and being non-medically switched by her insurance. She developed neuropathy as she fought with her insurance to get back on Cosentyx. She urged the board to consider access issues.
Discussion
The board discussed adding a high-level summary based on the rubric to the first few pages of each packet to help in decision-making for each drug. The board chair pointed out that Cosentyx had a high prior authorization percentage, compared to other drugs that have percentages around 36%. She said that the Office of Inspector General released a report saying that prior authorization impacts consumer access to drugs, which concerns her.
Creon drug review
The board chair highlighted a significant delta between the WAC and an increase in pharmacy acquisitions cost, which could cause access issues. One board member asked whether Creon was only used for cystic fibrosis or whether it had other uses. He was surprised that it has a high Medicare population. Staff said that there is no off-label clinical use and she was not sure why Medicare has higher utilization.
Another board member offered that they had personally seen it prescribed for other patients, including pancreatic cancer patients, and that they had encountered many issues with prior authorization and step therapy. He was surprised to see that the packet said only 1% required prior authorization. Another third board member mostly saw other products being used instead of Creon. Staff said that the data was based on the data call from 11 carriers, and that there may be gaps based on which carriers reported information. This was also expressed in the public comment, both written and verbal.
The next Oregon PDAB meeting will be held on September 17, 2025.
Washington
Washington PDAB Advisory Group Meeting, August 12, 2025
Drugs Chosen for Affordability Review
The purpose of the meeting was to discuss the next steps of the affordability review process with the advisory group. The board began with Enbrel and Xtandi. Additionally, they discussed expanding the advisory group to include two specialists on those drugs, as the statute requires.
Affordability Review Process & Needs from the Advisory Group
Recruiting Supplemental Advisory Group Members
The advisory group has followed up on the proposal for a stable core advisory group with an additional supplemental advisory group by opening applications for supplemental members. At the time of the meeting, two clinicians and one patient advocate had applied. They are waiting for more applications and reviewing them for supplemental advisors. Members of the advisory group suggested people and places to reach out to to get more applications for these positions.
Sharing Patient / Patient Advocate Surveys
A staff member presented surveys to collect information about the drugs selected for affordability review. One survey is targeted at patients and caregivers; another is targeted at medical experts. The questions are similar but tailored to each audience. The staff member presented the surveys in more depth and asked the advisory group for opinions on each.
The patient survey is designed to gather information about patient demographics, past and current medication use, and cost burdens for drugs selected for affordability review. Advisory group members noted that the survey was quite long, and asked staff to include questions about insurance hurdles, how the drugs are typically acquired by patients, and whether patients have trouble paying insurance premiums. A member said that patients may not know the difference between the types of coupons they are applying for the drug, but that they like that the question is included. Another asked staff to ensure that the survey is accessible with screen readers and that it defines more advanced terms.
They asked about the length of the public comment period for the survey. Staff replied that they were finishing internal reviews, and will get the draft posted as soon as possible. The staff also ask advisory group members to send ideas about how to get this survey around to patients.
The expert survey was very similar, asking questions about demographics, practice setting, and affordability for patients. Feedback included asking staff to add “specialty pharmacy” as an option for the suppliers of drugs and to add Medicare Advantage plans as an insurance option. Group members questioned whether the 340B questions were necessary. If they were, they asked staff to consider questions about sliding scale payments and to add hospital outpatient care as an additional option.
The staff asked for suggestions about how to distribute the survey.
Discuss How the Advisory Group Will Contribute to the Affordability Review
The advisory group talked about when they would contribute to the process. They spoke about an outline for the report, which would follow previously presented information, but was not drafted yet. Staff suggested that the advisory group meet and talk about survey results or internal data collection.
The next PDAB meeting will be November 19, 2025.
Standout resources and coverage
Strategic Directions Rx published Diagnosing Medicaid Drug Overspend in the Commonwealth Part I: An Analysis of Virginia Medicaid Prescription Drug Spending 2017-2023, which examines managed care organizations and pharmacy benefit managers as major cost drivers in Virginia's Medicaid program.
Read the highlights or the entire report.