October 20, 2025: PSM leadership lends their expertise to scholarly journals
PSM Board Vice President Dr. Kenneth McCall was recently named an associate editor of the Journal of Pharmacy and Pharmaceutical Sciences, the official journal of the Canadian Society for Pharmaceutical Sciences. McCall is a clinical professor and chairs the Department of Pharmacy Practice at SUNY Binghamton’s School of Pharmacy and Pharmaceutical Sciences.
Executive Director Shabbir Safdar joined the editorial board of the Journal of Illicit Trade, Financial Crime, and Compliance, which plans to release its inaugural issue in December 2025.

Board Vice President Dr. Kenneth McCall

Executive Director Shabbir Safdar
Domestic News
A California woman was convicted a second time for killing someone with silicone injections. FDA warned another company to test excipients for diethylene and ethylene glycol.
A jury found Riverside County, California resident Libby Adame guilty of second-degree murder and practicing medicine without a license after she administered the silicone injections that killed actress Cindyana Santangelo in March 2025. This is the second prosecution for Adame. She and her daughter, Alicia Galaz, were convicted of involuntary manslaughter in 2024, when another client died of an embolism after receiving these injections.
The U.S. Food and Drug Administration (FDA) has not approved silicone injections for body contouring and warned consumers against the dangerous practice in 2017 and 2021.
Gastonia Police and the North Carolina State Board of Investigation seized more than 10,000 fentanyl pills and two portable pill press machines when they shut down a fentanyl lab in Stanley.
Regulators protecting patients in the news
The FDA posted a warning letter rebuking New Jersey-based Health and Natural Beauty USA for failing to itest high-risk components for diethylene or ethylene glycol contamination before using them in its over-the-counter products. The agency is enforcing updated guidance issued in 2023 after hundreds of Gambian, Indonesian, and Uzbek children were fatally poisoned by cough syrup contaminated with the solvents.
The Ohio Board of Pharmacy suspended eight additional clinics for distributing dangerous medicines, including a Chagrin Falls medical spa where the owner began emptying a cabinet of expired topicals and drugs into a plastic bag during the inspection. The Board’s suspension notice lists substantial storage and recordkeeping issues, and alleges that the spa acquired medicines from unlicensed sources, compounded medicine without proper equipment, and injected a patient with a solution of purported semaglutide and expired bacteriostatic water.
Read about the need for better med spa regulation.
Patient safety issues in the GLP-1 space
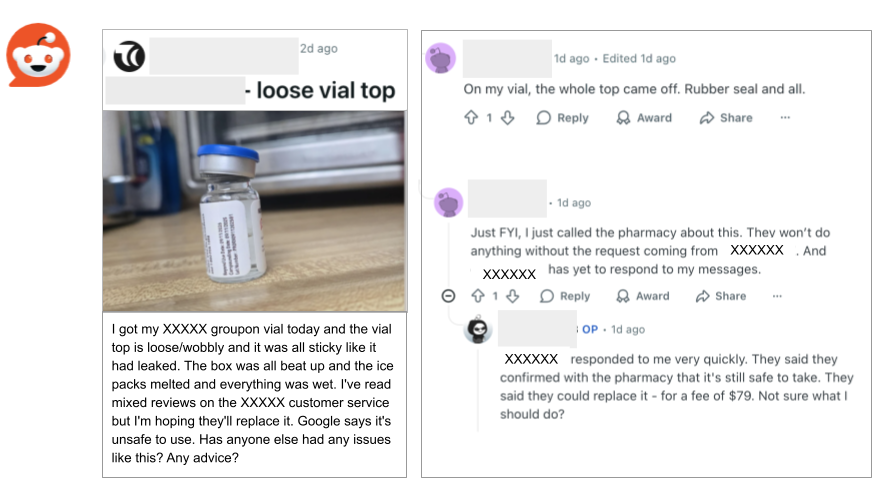
Buyer Beware: Two Reddit users who received defective vials of tirzepatide from an online retailer’s Groupon promotion aren’t likely to get fair replacements. Their vials weren’t properly sealed, but the compounding pharmacy that made them referred them back to the company that had posted the Groupon. One user said that the online seller told them that the damaged vial was safe, but FDA guidance says that vials with damaged seals are vulnerable to contamination. Injecting yourself with their contents could directly introduce dangerous microbes into your bloodstream. In the past, that has caused illness and deaths.
The Groupon company also said it would charge $79 to replace the vial.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
International News
Novo Nordisk can block U.K. websites selling fake Ozempic. Ireland seized fake weight-loss injections made with insulin. France is fighting an uptick in GLP-1 counterfeits.
England’s High Court ruled that Novo Nordisk has the right to force internet providers to block access to websites that are selling counterfeit or unregulated forms of semaglutide.
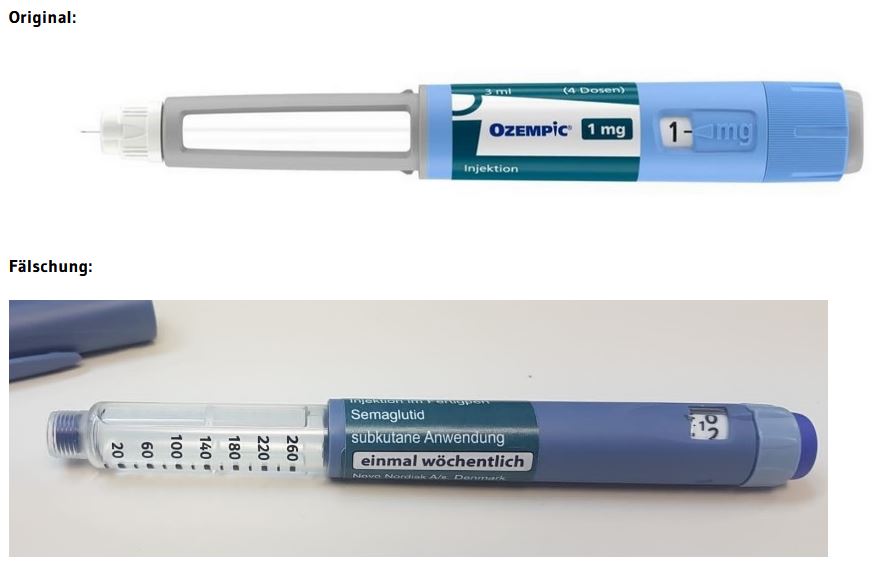
Ireland’s Health Products Regulatory Authority reported seizing a package of counterfeit Mounjaro pens, some of which were filled with insulin. Similar counterfeits have been found all over the world, and led to hospitalizations in Austria in 2023.
The Health Ministry in Yucatan, Mexico, reported that two pediatric hospital patients had suffered adverse reactions after receiving counterfeit or adulterated rituximab. The incident is another reminder that Americans seeking less expensive medicine in Mexico may encounter dangerous counterfeits.
France’s National Agency for the Safety of Medicines (ANSM) is cracking down on illicit sales of purported GLP-1 products. After seeing a sharp increase in online promotion of the drugs, the agency is taking legal action against approximately ten merchant websites for selling counterfeit GLP-1s. It also reported seizing unapproved GLP-1 patches containing microneedles designed to be applied to the skin.
Authorities in Vietnam arrested a TikTok influencer who allegedly sold counterfeit weight-loss pills and “collagen capsules” that contained banned substances such as sibutramine and phenolphthalein.