February 9, 2026: PBMs are the target of the spending bill and the FTC investigation
Major Stories
The federal spending package that ended January’s government shutdown will prohibit PBMs from linking rebates to drug companies’ list prices. The FTC, also addressing PBMs, found that they inflated the prices of generic drugs.
The recently signed $1.2 trillion spending package will prohibit pharmacy benefit managers (PBMs) from linking rebates to drug companies' list prices and will be required to pass all of the rebates to plans and sponsors. The legislation will also require PBMs to submit semi-annual reports on drug spending, rebates, spread pricing, formulary rationale, and benefit design.
Federal Trade Commission (FTC) investigators reported that PBMs inflated the prices of specialty generic drugs, including medications for heart disease and cancer, beyond their costs of acquisition, earning more than $7.3 billion in revenue from 2017 to 2022. FTC staff also found that PBMs reimbursed affiliated pharmacies at higher rates than unaffiliated independent pharmacies for almost every one of the generic drugs that they analyzed.

The Drug Enforcement Administration announced the seizure of more than 200 domains linked to an India-based transnational criminal organization working from the United States that is allegedly responsible for at least six fatal and four non-fatal overdoses.
GLP-1s in the news
Hims & Hers sparked a major legal and regulatory battle when they announced a compounded pill version of Novo Nordisk’s weight-loss drug Wegovy, prompting Novo to accuse the telehealth firm of selling an unapproved and unsafe knockoff. FDA general counsel escalated scrutiny by referring Hims to the Justice Department and warning that compounded GLP-1 drugs cannot be marketed as equivalents to FDA-approved medicines. The dispute culminated in Novo filing a lawsuit to permanently block Hims from selling versions of its drugs, despite Hims saying it would halt the pill rollout after the backlash.
The FDA has found counterfeit Ozempic in the U.S. drug supply three times since 2023. (U.S. FDA)
After losing weight on legitimate Ozempic, a U.K. woman nearly died after taking what she thought was the same drug, which she had acquired from a Facebook seller when supplies became harder to find. The counterfeit pen was actually filled with insulin, sending her into a diabetic coma.
The Guardian reported that hidden-market sellers are using WhatsApp and Telegram “giveaway” competitions to promote weight loss drugs, like the still unapproved Retatrutide, obtained outside of the legitimate supply chain.
Domestic news
Drug manufacturers in Canada dispute Florida's claim that they are negotiating the state's importation program, and two major sentencings in cases related to counterfeit drugs.
Canadian drug manufacturers and distributors say they are not negotiating with Florida on its plan to import cheaper prescription drugs from Canada, contradicting claims from Florida that they are “working diligently to negotiate with Canadian manufacturers.” Innovative Medicines Canada has “not spoken to Florida about this, and to our knowledge, neither have our members." The program, approved by the FDA in 2024, has yet to ship a single drug, despite costing the state $80 million.
A federal judge sentenced a Texas-based fentanyl trafficker to life in prison for leading a large distribution ring as part of the multi-agency “Operation Top Shelf.”
The owner of Incognito Market, one of the largest dark-web drug marketplaces, was sentenced to 30 years in federal prison. The site facilitated more than $105 million in illegal narcotics sales worldwide and was linked to at least one death.
Regulators protecting patients in the news
The FDA issued two recalls last week, the first for Akkarco LLC’s honey-based energy supplement that contained undeclared tadalafil, which is a prescription-only drug. The second recall, for Abbott’s FreeStyle Libre 3 and 3 Plus glucose sensors, was issued after they were found to give falsely low readings. This problem is linked to 860 serious injuries and seven deaths.
The FDA also sent out two warning letters.
- An Indian manufacturing facility was cited for inadequate investigations of product quality complaints, poor root-cause analysis, and major failures in cleaning, maintenance, and validation of shared manufacturing equipment that posed cross-contamination risks. The company, Cohance, has temporarily suspended drug manufacturing for the U.S. market.
- A Nevada manufacturing facility was warned that its drug products were adulterated due to weak quality unit oversight, mislabeled products following API changes, lack of process validation, and an inadequate stability program to support expiration dates. Kirkman has also ceased U.S. drug production.

Akkarco LLC's energy support supplement. (U.S. FDA)
In Ohio, the Board of Pharmacy issued two new summary suspensions in early February:
- At GRT Performance Methods, a Toledo med spa, inspectors found dozens of illegal injectable drugs sourced from an unlicensed foreign supplier, many expired or improperly stored, and admitted by the owners to be sold directly to customers without guidance. One co-owner told investigators they order the drugs online and sell them to people “who come in looking for them,” while another said buyers have to “figure it out.”
- Board inspectors found dangerous drugs from unlicensed, non-FDA-approved sources at Phoenix MD in Cincinnati. The drugs, including tirzepatide and foreign Botox, were found alongside expired medications, unlabeled syringes, and poor storage controls. Inspectors also discovered patient-specific drugs from 2018 still in active stock and additional unlicensed products openly displayed at the clinic, underscoring what the Board described as systemic failures in drug handling and compliance.
Legislation
In the U.S. Senate, Jim Banks introduced the bipartisan SAFE Drugs Act, which would close loopholes allowing mass production of compounded versions of approved drugs, require greater FDA reporting and inspections, and strengthen limits on when compounded drugs can copy FDA-approved medicines.
At the state level, Maine advanced legislation to provide staffing support for its Prescription Drug Affordability Board, while West Virginia introduced bills to formally establish its own affordability board, study pricing controls such as upper payment limits and bulk purchasing, and create a state-run wholesale Canadian drug importation program pending federal approval.
Illinois introduced HB1443, which would create a Health Care Availability and Access Board empowered to review high-cost drugs and impose upper payment limits tied directly to Medicare’s Maximum Fair Price, with enforcement authority granted to the state attorney general.
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, drug importation, and med spas.

Learn why UPLs are a bad solution for drug affordability in the U.S.
International
Fight the Fakes spoke at the WHO Executive Board meeting. New analysis suggests that Europe is underestimating the threat of online marketplaces for illicit drugs, and counterfeit anti-rabies vaccines in the Philippines.
At the 158th World Health Organization (WHO) Executive Board meeting, the Fight the Fakes Alliance urged stronger global action against substandard and falsified medical products, emphasizing the need to include technical experts and people with lived experience in WHO working groups.
A new analysis warns that Europe may be underestimating the public health threat posed by booming online marketplaces for illicit and counterfeit pharmaceuticals, where fake and diverted medicines are sold alongside street drugs. Researchers highlighted the rise of highly potent synthetic opioids disguised as prescription drugs, the role of digital platforms and courier networks in enabling transnational crime, and regulatory gaps that leave consumers vulnerable to overdose, poisoning, and death.
In the Philippines, the FDA warned the public about counterfeit anti-rabies vaccines circulating in the market, cautioning that fake versions of anti-rabies serum (equine) pose serious health risks.
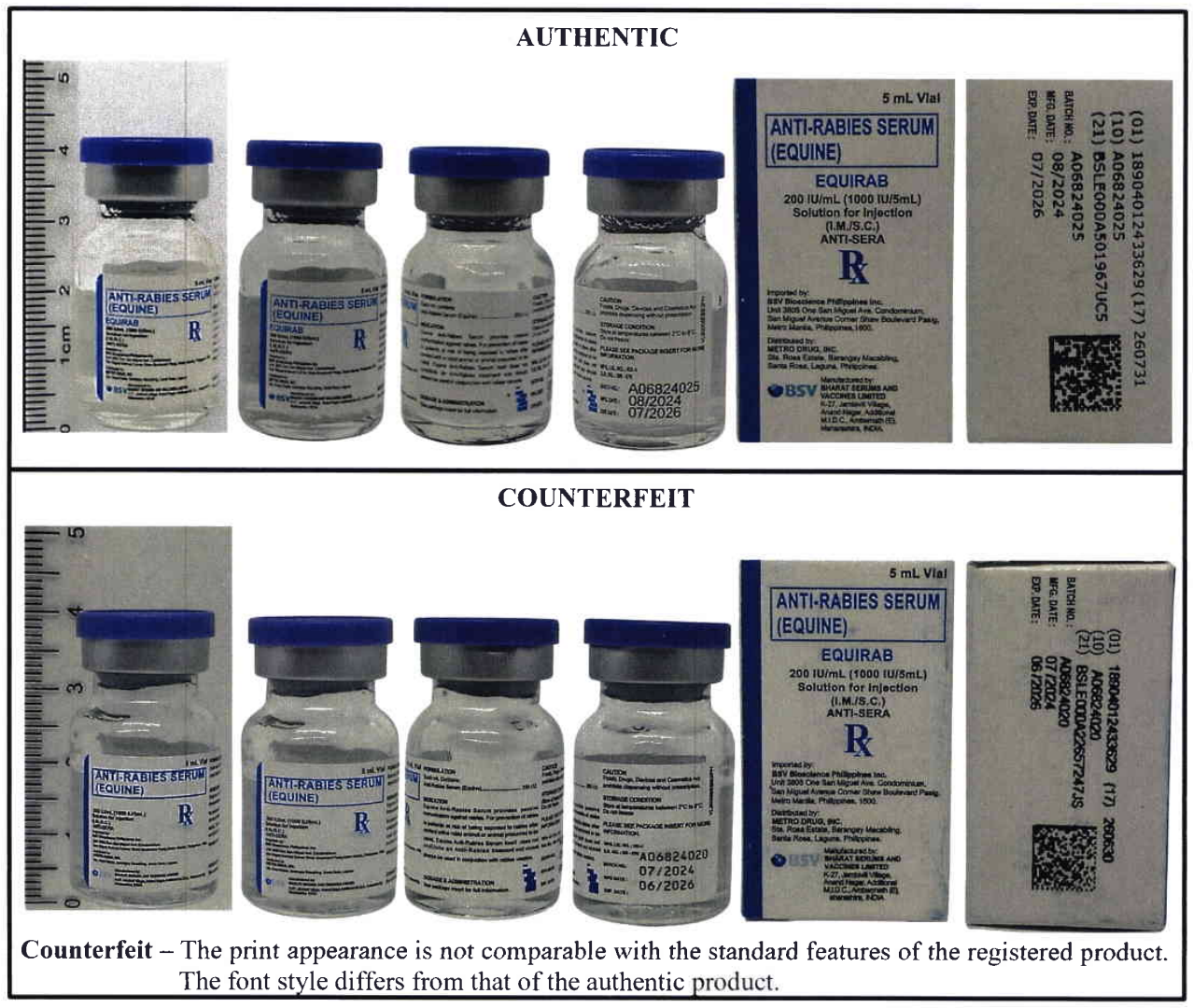
Authentic vs counterfeit anti-rabies vaccines. (Ph FDA)