Prescription Drug Freight Fraud Report, May 2025
What if your diabetes medication was manufactured at a gas station? Or your blood thinners were mislabeled and shipped in from an unverified address in Colombia?
In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect, including hardware stores and shopping plazas. In our analysis of a sample of shipments of imported semaglutide, tirzepatide, antibiotics, and apixaban in March, we found 54 examples of shipments from facilities that are not in the FDA’s Drug Establishments Current Registration database. We identified 20 of these flagged shipments as particularly suspect, from semaglutide made at a gas station in Canada to Lysergide, also known as LSD, labeled as an antibiotic and granted entry into the United States.
Truthfully, none of these were probably manufactured in those places, but the smugglers are so confident in their success rate that they outright lie on the shipping manifests.
Freight shipments that are declared with illegal, unregistered manufacturers are the ones we know about. What we don’t know about are hidden contraband medicine shipments inside other, legitimate shipments. The public is familiar with the smuggling of illicit drugs such as cocaine hidden in other shipments (thanks Narcos!), but it also happens with counterfeit therapeutic medicines.
Each day, dedicated agents from Customs and Border Protection and the Food and Drug Administration do the best job they can, with the resources they have been given, to keep dangerous products out of the U.S. PSM applauds their efforts and often highlights their successes to bring attention to the difficult but important jobs they do.
Our report on dangerous foreign shipments of semaglutide, tirzepatide, antibiotics, and apixaban in March 2025 is one possible metric to help improve the system that keeps Americans safe. When CBP and FDA have the tools and legal support to do their jobs well, all Americans win.
This isn’t the only way unsafe medicines get into the U.S.
Every year, 1.3 billion packages enter the U.S. in the small package mail and arrive without any electronic data because they are valued at less than $800 and categorized as “de minimis.”
Though policy around de minimis shipping is changing quickly, it is the easiest way for illegal and unlicensed fake online pharmacies to ship uninspected and unsafe medicines to Americans.
Our research process
We began our research by gathering all the March 2025 entries for semaglutide, tirzepatide, antibiotics, and apixaban in the FDA’s Imports Entry Data Search(1). Then we cross-referenced facilities associated with imports of semaglutide, tirzepatide, and apixaban against the FDA’s Registered Drug Establishments list using the manufacturer FEI number, the manufacturer name, or the manufacturer address. Through this process, we found 27 shipments of semaglutide, seven shipments of tirzepatide, and at least five shipments of apixaban, tied to facilities that are not FDA-registered.
For every shipment across our four types of drugs, we looked up the shipment ID in the Import Trade Auxiliary Communications System (ITACS) to examine a more detailed manifest of what else was included in each container, validate declared quantities, and uncover inconsistencies. This helped us better understand the context of the semaglutide, tirzepatide, and apixaban shipments. You can learn more about ITACS in this FDA industry presentation.
In the antibiotics category, the focus was different: we looked for classification mismatches. When we looked at the more detailed manifests available through ITACS we found 15 cases where compounds labeled as “antibiotics” were not antibiotics at all.
(1) We did not do a comprehensive search of every single import in the database, just four drug categories we have seen a lot of fraudulent activity around.
What we found
For the 54 suspect shipments across all categories, we took an additional verification step: investigating the supposed manufacturers. Imports of medicine are accompanied by a manifest that includes a space for the manufacturer of the product, and these can be used to identify shipments of unsafe products quickly if the declared manufacturer isn’t an FDA-registered facility. Using Google Street View, business registry databases, and local pharmacy boards, we traced the physical addresses and legitimacy of listed manufacturers. 20 of these 54 cases stood out as especially egregious and illustrative of oversight failures that could have been prevented from entering the U.S. and endangering American patients.
Here are some of the most alarming examples:
“Semaglutide manufacturer”: Bridlewood Home Hardware
This shipment of semaglutide was supposedly made at this hardware store. We were shocked that it was openly listed in ITACS as “for personal use.” The location is not an FDA-registered manufacturer.
Furthermore you can see from ITACS that additional documentation was submitted to help clear this product.
We have to wonder what was in the additional submitted documents that made it ok to enter a non-FDA approved medicine into the country.
We don’t actually believe that any semaglutide was manufactured here legally or illegally, merely that the smugglers put a completely unbelievable business as the manufacturer.
“Mounjaro manufacturer”: An individual with a grocery store address
One shipment of tirzepatide (Mounjaro) was listed as manufactured by an individual with an address in Calgary, Alberta. The individual is not a registered FDA facility, and the address is from a Sobeys, which bills itself as “Canada’s Family Grocery Store.”
While we can’t really tell from Google Maps what’s inside, we are confident this grocery store is not a facility producing Mounjaro for Lilly.
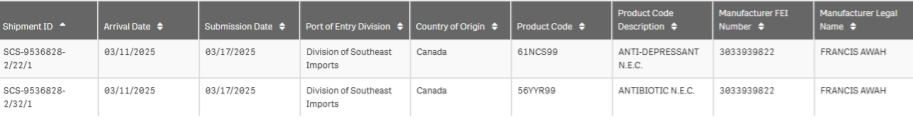
Controlled substances declared as antibiotics and anti-depressants
This shipment contained an anti-depressant and an antibiotic, according to the top-level FDA Imports database. The manufacturer’s name is listed as the shipping and logistics supervisor at Toronto Research Chemicals, a company that appears to have an office or facility at the address in the manifest.
Toronto Research Chemicals is not an FDA-approved drug establishment site. Any medicine or ingredient for medicine in the shipment should have been refused because it is not a site registered with the FDA.
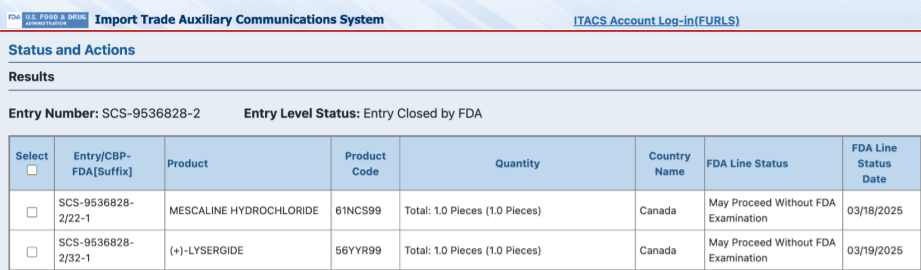
However, we were taken aback when the detailed manifest showed that instead of an antibiotic and an anti-depressant, it was actually a shipment of mescaline and lysergic acid (LSD), both Schedule I controlled substances.
This is a form of “code fraud” we see in shipments frequently. It probably works because CBP and FDA scanners are only looking at the top level code, not the product descriptions in the shipping manifests.
This shipment was granted entry with minimal challenge, and it highlights how much data is already available to catch fraud and stop unsafe shipments.
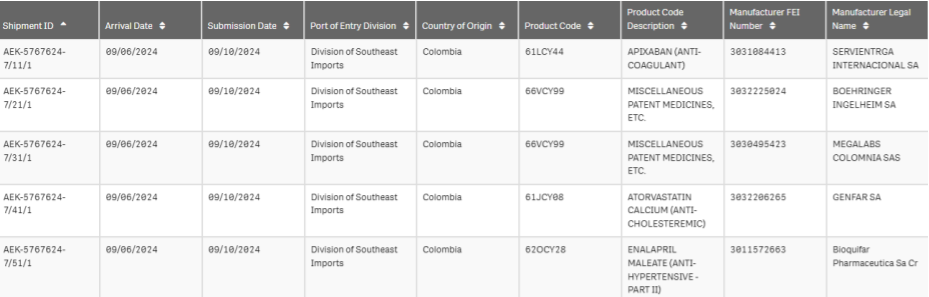
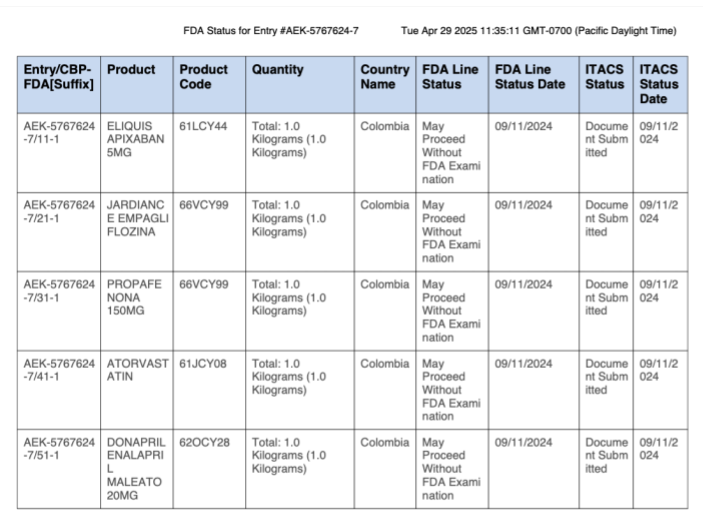
Five kilograms from Colombia (September 2024)
It didn’t happen in March, but we wanted to highlight a dangerous shipment we noticed while pulling data from back in September 2024. It was five kilograms of therapeutic medicines all supposedly made in Columbia, and none of them were at FDA-registered facilities.
FDA Imports record for 5-part shipment from Columbia
FDA ITACS data for 5-part shipment from Columbia
This is the location in Bogota where the apixaban was supposedly manufactured.
The manufacturing address of this apixaban anticoagulant looks quite residential.
This shipment of apixaban, an innovative, blockbuster anticoagulant, came in bulk from a logistics company in Colombia with no registered drug manufacturing credentials. And it made it through.
There are two disturbing facets of this shipment:
- Apixaban is a valuable new innovative medicine. Patients use it to prevent strokes without requiring frequent blood tests and dosage adjustments with older treatments like warfarin. A treatment failure due to a counterfeit could have catastrophic consequences for the patient—like a stroke.
- A plastic bottle with 30 5 mg pills weighs about 20 grams. Each of these shipments, including the apixaban, was for 1 kilogram. This shipment represents approximately fifty bottles of unsafe medicine made in a facility never inspected by the FDA.
None of the listed manufacturers on the manifest were entities registered with the FDA.
Does this building contain a Boehringer Ingelheim manufacturing facility? (Cl. 116 Sur #7-34, Bogotá, Colombia)
Policy recommendations
U.S. patients are protected from counterfeit medicines by the highly regulated, closed, secure drug supply chain. When criminals ship prescription medicine made in facilities unknown to and unsuspected by the FDA, American patients are put at risk.
The findings from our March 2025 analysis reinforce the urgent need for stricter customs enforcement and improved interagency data sharing to flag and hold questionable imports before they can enter the U.S. and endanger American patients.
We suggest that CBP, FDA, and all import inspectors in any agency working on medicine adopt the following measures to protect American patients:
- Refuse shipments of medicine that aren’t from FDA-registered facilities / addresses
This seems obvious, but medicine is not allowed in our drug supply unless it was made in an FDA-registered facility. Shipments of medicine where the manufacturer’s name and address don’t match the FDA’s registered facilities database should be refused. - Check for product code fraud; search descriptions of medical products to see if they match
It seems shocking to us that smuggling in a Schedule 1 controlled substance is as easy as using the code for antibiotics while declaring the description to be LSD. This code fraud should be easy to detect; it doesn’t need complex AI technology.