June 2, 2025: FDA continues to protect Americans from unapproved and contaminated medicine
Domestic News
The FDA warned companies over the sale of an unapproved medical device and contaminated eye medicines, and disbarred a Texan who sold illegally imported pharmaceuticals.
The U.S. Food and Drug Administration (FDA) posted warning letters it sent to a German company selling an unapproved medical device and a New Jersey manufacturing facility for sterility issues in the production of eye lubricants, which were the subject of a recall in January 2024.
The agency also barred Evan Asher Field from importing any drug into the United States for a period of five years. In October 2023 the New Braufels, Texas resident received a five-year prison sentence for importing bulk quantities of various drugs, including synthetic opioids and benzodiazepines, reselling them to U.S. residents for “research purposes only.”
A federal judge in South Carolina sentenced York County residents Javaris Latrey Johnson and Thomas Anthony Perry to prison sentences of more than twelve years and eight years, respectively, for their parts in a drug conspiracy that manufactured and distributed fentanyl pills. Agents discovered the men, two conspirators, several pill press machines and more than 30 kilograms of illicit drugs— including over 150,000 fentanyl pills—in a trailer in October 2022.
Legislation
PSM monitors new state legislation weekly in the areas of pill presses, prescription drug affordability boards, and drug importation. Follow our tracking of state legislation in these places by clicking on the topic links above.
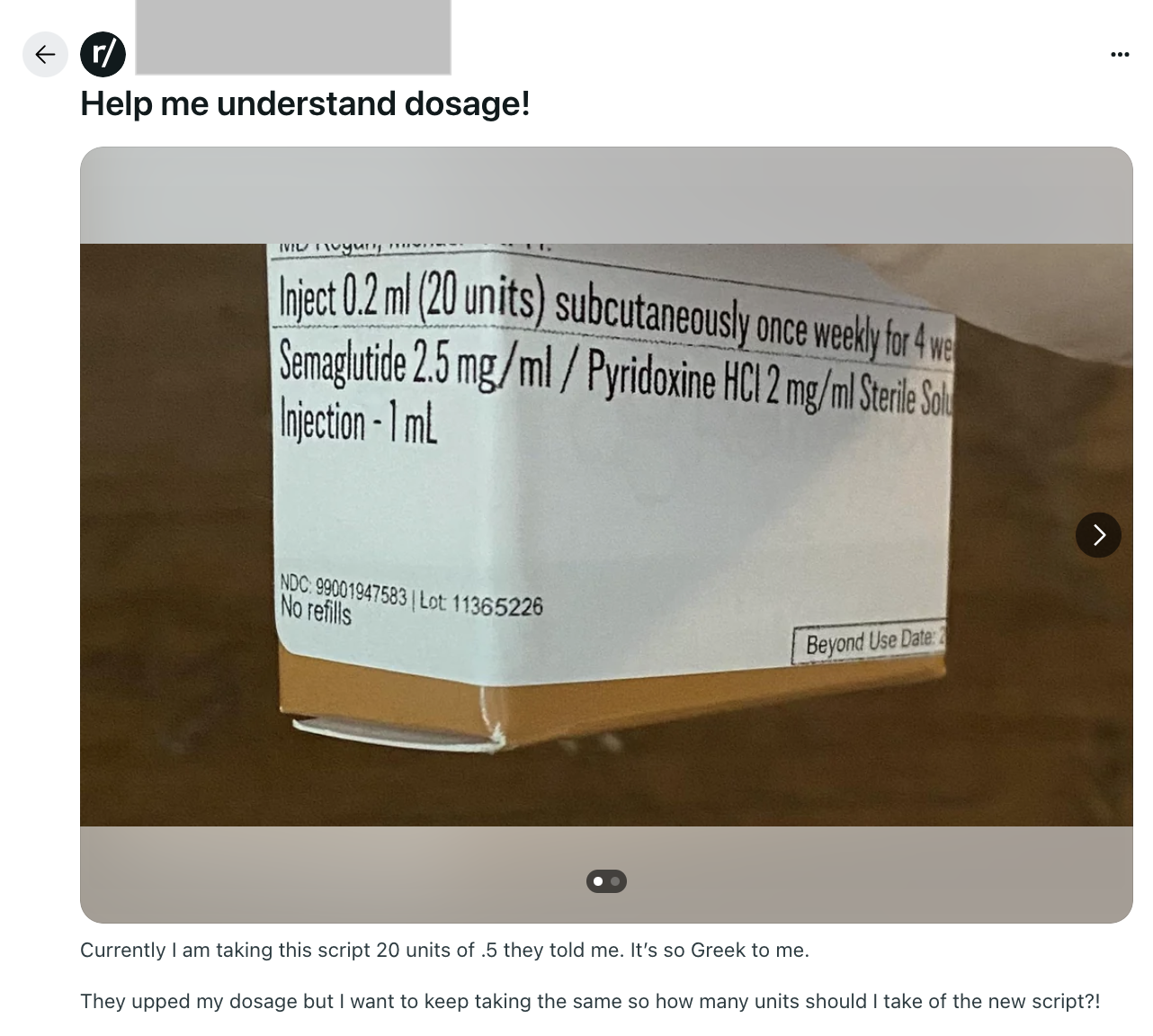
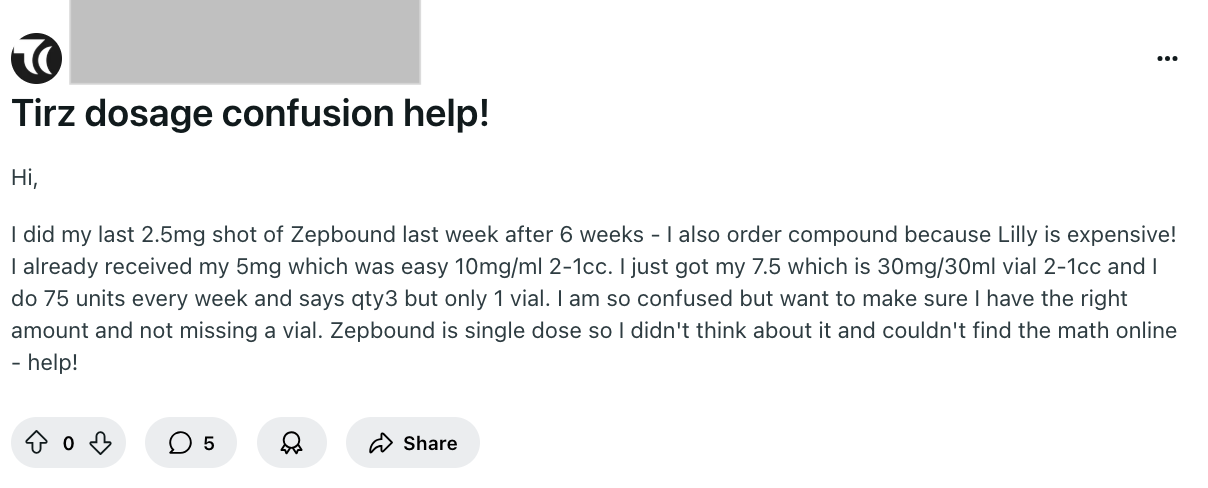
Patient safety issues in the GLP-1 space this week
Now that the FDA has declared semaglutide and tirzepatide no longer in shortage, compounding pharmacies are not supposed to be mass producing them. However, patients are still injecting these drugs, and this sampling of posts since May 28th shows they don’t understand how to calculate the right doses. This is a real danger to patients, as overdosing can cause side effects ranging from nausea and vomiting to dehydration or hypoglycemia.
International News
A Welsh man was sentenced for making and distributing poisonous diet pills. Substandard and counterfeit medicines were reported in India, Vietnam and Nigeria.
A former fentanyl dealer from Wales received a three-year jail sentence for making and selling diet pills made with 2,4-Dinitropheno (DNP). DNP, an industrial chemical that was marketed for weight loss in the 1930s, even though it caused dehydration, cataracts, liver damage, and death. This case recalls the prosecution of New Jersey doctor WIlliam Merlino, who received a three-year sentence for making and selling DNP pills in June 2023.
India’s Central Drug Standard Control Organization warned about a batch of nandrolone decanoate, an anabolic steroid, that failed quality checks.
The Drug Administration of Vietnam is investigating a pharmacy in Hanoi that was found selling subpotent diabetes medication and drugs from unknown sources, including products bearing labels familiar to Americans: Crestor, Janumet, Nexium and Plavix.
Nigeria’s National Agency for Food and Drug Administration and Control seized expired and relabeled injectable medicines, including antimalerials, antibiotics and analgesics during the raid of an illicit drug manufacturing facility in Delta State.