Prescription Drug Affordability Board Activity, May 2025
Activities Summary
Colorado: Colorado's PDAB met on May 23 to approve the Data Submission Guide and begin rulemaking around an upper payment limit for Enbrel.
Oregon: At its May 21 meeting Oregon's PDAB discussed the timeline and process for affordability reviews and approved the generic drug report. One board member proposed looking beyond upper payment limits for ways to lower drug prices.
Washington: On May 21, Washington's PDAB reviewed policy changes around drug selection and data collection. The board also considered choosing the top five drugs from short lists of specialty and non-specialty drugs for public comment in July.
Activity by State
Colorado
Colorado PDAB Meeting, May 23, 2025
The board heard updates related to the Colorado legislative session, which ended earlier in May, and the acknowledgement of numerous healthcare-related bills that may be discussed in future PDAB meetings.
- Harry Gewanter raised concerns about supply issues with price controls using an analogy about derision toward the French government in 1947 when government enforced price controls caused empty shelves. He also brought up the example of Arkansas CVS closures. The board made no comment.
- Ranier Simons, on behalf of CANN, raised concerns about the lack of detail in the cost-benefit analysis posted in April.
- Amy Goodman, with the Colorado BioScience Association, raised concerns about the integrity of data underpinning affordability decisions; UPLs not impacting what patients pay, rather what upstream purchasers pay; and how affordability decisions impact patient access. The board and staff responded that they have slowed the process considerably, and are trying to take their time to do this properly.
- Tiffany Westrich, on behalf of EACH, raised concerns about how, with such limited time, there should be more resources put towards revisiting the data used to rule Enbrel unaffordable.
- Primo Castro, with the Biotechnology Innovation Organization, raised concerns about cost-benefit analysis, including whether the proposed rule would justify its cost, particularly related to its lack of quantifiable data.
- Scott Burns of Johnson & Johnson asked the board to cancel UPL rule-making on Stelara, citing erroneous data and newly developed biologics and biosimilars, a factor that excluded other medicines from being subject to affordability review. The board replied that they would take another look.
- Brett Johnson, on behalf of Amgen, wanted to register discontent with board actions related to Enbrel.
To conclude public comment, staff acknowledged cooperation with the Oregon PDAB team and said they will take steps to have a conversation with their Director.
Written comments may be found in the meeting materials.
General Assembly Report Discussion
The board discussed adding a consumer advocate and considered the morality, the selection process of the representative, and the logistics of adding a consumer advocate to the board.
The board passed a motion to recommend that the legislature consider the benefits of adding a qualified consumer advocate to the board.
Board approved the Data Submission Guide (DSG)
A staff member introduced the DSG and its purpose for providing guidance for stakeholders. They spoke about meetings with the Prescription Drug Affordability Advisory Council (PDAAC), where they got recommendations about how to improve the DSG. The following are some changes that were implemented:
- Provide a glossary of common terms for stakeholders
- Allow staff and board contractors to review any data submitted with dollars-per-quality adjusted life year (QALY), and
- Clarify the confidential data submission process by
- including step-by-step instructions on communication and data confidentiality and
- clarifying how stakeholders can join the executive session during a rulemaking hearing.
These changes were approved after discussion.
The board continued discussion from the April meeting about the Enbrel affordability review, which largely focused on the issue of miscategorized data in the APCD. They updated the numbers and charts and presented updated patient counts and out-of-pocket costs. The board approved the addendum to the Enbrel affordability review and passed a motion to approve rulemaking to set an upper payment limit.
The rulemaking hearing began by reviewing the process and how witnesses can testify. Staff presented data about Enbrel, including how and why it is used, cost metrics, acquisition costs, federal government payments, and out-of-pocket costs. The board moved to an executive session to discuss confidential trade secrets and proprietary information, and to get legal advice.
After further discussion of metrics related to Enbrel, they decided to postpone witness testimony until the next PDAB meeting on July 11, 2025.
The next PDAB meeting will be on July 11, 2025.
Oregon
Oregon PDAB Meeting, May 21, 2025
John Murray declared a conflict of interest and disclosed that he had spoken to the board chair and executive director to propose expanding the PDAB’s discussion to ways prescription delivery systems have saved money in other states. He noted that public sentiment presumed that conversations about affordability would lead to UPLs, and Murray wanted to push the PDAB to be different from others.
Executive director’s program update
Senate Bill 289 passed in both committees and will head to the governor's office. The changes include:
- Revising the number of affordability reviews from “9 drugs a year” to “up to 9 drugs a year”
- Removing the requirement that the Department of Consumer and Business Services provide the board with prescription drugs each calendar quarter
- Replacing the annual generic drug report requirement with a new provision that relevant content would be incorporated into the affordability review reports
- This bill would not go into effect until January 2026.
Legislation Update
HB 2057: Deals with 340B program. Awaiting scheduling for floor vote.
HB 2149: Create a new licensure and regulatory program for PSAOs. In the House Rules Committee.
HB 2385: Relates to 340B program and prohibits drug manufacturers from interfering with 340B acquisition process. Awaiting Senate floor vote.
HB 3212: Significantly expands regulation of PBMs. In the House Rules Committee.
SB 533: Deals with 340B program. Awaiting scheduling for floor vote.
- Tiffany Westrich-Robertson [AiArtritis, Ensuring Access through Collaborative Health (EACH) & the Patient Inclusion Council (PIC)] - She expressed appreciation for the earlier conversation about the Oregon PDAB being innovative in its approach to lowering the costs of medicines. They also expressed appreciation for adding patient advocacy groups to the patient collection data, but are concerned about the robustness of this data. She encouraged the Board to keep patient experience at the center of their discussion, and said a narrow focus on payer cost could miss the true impact on access to medicines.
- Bill Schmidtknecht (Patient Protector) shared the story of the loss of his son, who was priced out of his medicine. Schmidtknecht advocates for PBM reform.
- Derek Flowers (Value of Care Coalition) was concerned that the number of therapies to be reviewed and the proposed timeline would not permit all impacted parties to be taken into consideration. He said that there was potential for each drug to only be given 20 minutes of deliberation. He also commented that UPLs could be authorized in the future, and these discussions would lay the groundwork for potential public policy.
- Dharia McGrew (Pharma) brought up the board's pause in deliberation last year, saying that the reasons for the pause, including the desire to better define affordability, have not been solidified yet. She also expressed concern about the timeline for the amount of drugs being considered for affordability review.
- Brian Mayo (Oregon State Pharmacy Association) urged the board to take more actionable steps by learning from other states that have successfully implemented cost-saving strategies. Mayo specifically called on the elimination of spread pricing by PBMs to improve patient access.
Board discussion on timeline, process, and voting methodology for affordability review determinations
The board is troubled by the number of drugs currently on the list for consideration and decided that voting on which drugs will be considered for review will take place in November, rather than voting on which drugs will advance after each month’s presentations.
Board review and vote on final generic drug report
Staff walked through the generic drug report, highlighting the changes made, for board comment. Assuming minor changes would be made, the board passed the motion to send the report to the Oregon legislature.
- Lindsey Silva (a mother and primary caregiver to someone living with cystic fibrosis, commenting on Creon) spoke about her daughter's experience with switching insurance and needing to prove failure on another drug before being allowed to switch back to Creon. She opposed reviewing Creon because she wanted to ensure her daughter’s access.
- Zach Lynkiewicz (HIV and Hepatitis Policy Institute) did not believe that HIV medications, including Odefsey, should be a part of the affordability review process. He cited substantial programs in Oregon that ensure access and expressed concern that the affordability review and the prospect for UPLs could affect insurers' decisions.
- Lucy Thornehaven (National Psoriasis Foundation, commenting on Humira) talked about her experience as a patient with Crohn's Disease. She is concerned about creating scenarios that would prompt insurers to change formularies or restrict access to critical drugs.
- Mary Jo Strobel (American Partnership for Eosinophilic Disorders) urged the board to consider limited FDA treatment for eosinophilic esophagitis and to ensure that Oregonians have equitable access to care.
- Ranier Simons (Community Access National Network) asked the board to exclude Odefsey from review to ensure access.
- Silas Martin (Johnson and Johnson) asked the board to exclude Tremfya and Xarelto from the affordability review. Martin said that PBMs do not pass cost savings onto patients, and reiterated concerns about the number of drugs in the review process. Martin noted that Johnson and Johnson provides various support programs to enhance patient access to Tremfya and Xarelto. They also noted that Xarelto is subject to a maximum fair price as part of the Inflation Reduction Act.
Oregon's next PDAB meeting will be June 18, 2025.
Washington
Washington PDAB Meeting, May 21, 2025
Mike Neuenschwander gave an update about the status of the fifth board seat, which remains unfilled after MaryAnne Lindeblad resigned due to a new position as the acting director for the Healthcare Authority. Neuenschwander cited waiting on budgets and appointments to stabilize within the state’s Healthcare Authority before finding a new board member.
Neuenschwander then presented updates on PDABs in other states.
First, he spoke about a recent lawsuit around Colorado's drug reviews, which was dismissed after a judge ruled that Amgen has no standing to sue. The court upheld that UPLs would not directly regulate the wholesale prices that manufacturers charge, which cleared the way for Colorado’s PDAB to continue their effort. Other news in Colorado pertained to the Enbrel rule making hearing, which was scheduled for May 23, 2025. Colorado was expected to introduce rules and have data presentations from the staff.
Finally, he talked about Oregon’s PDAB meeting, also on May 21, 2025, and its public comment period, which will gather feedback on prescription drugs and insulin products that have been selected for their affordability review.
Drug Selection Policy
Small changes were made to the drug selection policy as a result of a board member's request that the dashboard be sortable by ingredient. The change passed a board vote unanimously.
Data Dashboard Updates
Kelly Wu explained that four of the eligible drugs for review had been excluded because the National Drug Code (NDC) manufacturing data showed that they were repackaged.
An aggregation step has been added to the process of preparing the list of drugs available for affordability review: grouping drugs with the same ingredients from the same manufacturer or distributor will prevent products from being counted twice if they have multiple NDCs.
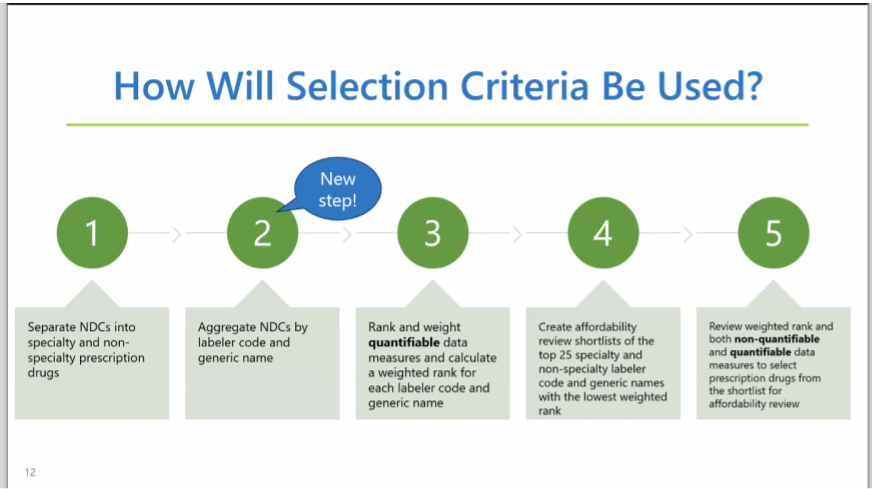
Kelly mentioned that Enbrel is the only drug to come out of Washington’s selection criteria that has also emerged in other states’ PDAB affordability reviews. During board questions, they concluded that this difference comes down to statute.
Finally, Kelly reviewed the dashboard, including new tabs that show changes to the data from this new step in the aggregation process. The board requested more updates to better sort and view data on the user's end.
How Many and Which Drugs to Select for Affordability Review
After discussion, the board made a preliminary decision to choose the top five drugs from short lists of specialty and nonspecialty drugs for public comment in July. After that, they will pick a smaller number of drugs to start assessing.
Both the core advisory group and the board seemed to support this recommendation, but no final decisions were made.
The board also agreed that Enbrel seems like an obvious first choice for the affordability review.
Next Steps for Drug Selection and Affordability Reviews
- Surveys and forms with stakeholders, as required by legislation.
- Meetings with stakeholders.
- Once the list of drugs is selected, add in drug-specific stakeholders, like advocacy groups and patients. This step is anticipated to take place from the July 15 meeting to sometime in the fall.
- Data collection is anticipated to be completed by the November 19 meeting.
- Make a report. Anticipated to be completed in early 2026.
- Continue these steps for other drugs.
- UPL conversations, which cannot take effect until 2027, per legislation.
Marina Suzuki presented on the data collection process, highlighting forms for manufacturers, payers, PBMs, and wholesalers. She addressed concerns about protecting confidentiality and proprietary information, particularly among manufacturers.
The board decided to shorten forms by excluding questions about foreign pricing and marketing, advertising, and lobbying costs, and making questions about R&D optional.
Finally, there was a discussion about ambiguity in the law regarding the use of QALY.
Oral comment was limited to Dharia McGrew of PHRMA, who mentioned that the limited time window may make it difficult for stakeholders to collect data needed to answer the questions on the form. She also raised concerns about a previously-mentioned problem with the lists that compare Washington's shortlist with other states, as well as a concern about aggregating drugs based on active ingredient.
Additional, written comments can be found in the meeting materials.
Washington's PDAB will next meet on July 15, 2025.



