The FDA found significant problems at Empower's new outsourcing facility
There has previously been some excellent reporting about patient safety concerns at Empower Pharmacy, a well-known compounding company that makes compounded GLP-1s. This latest FDA warning will not allay anyone’s concerns.
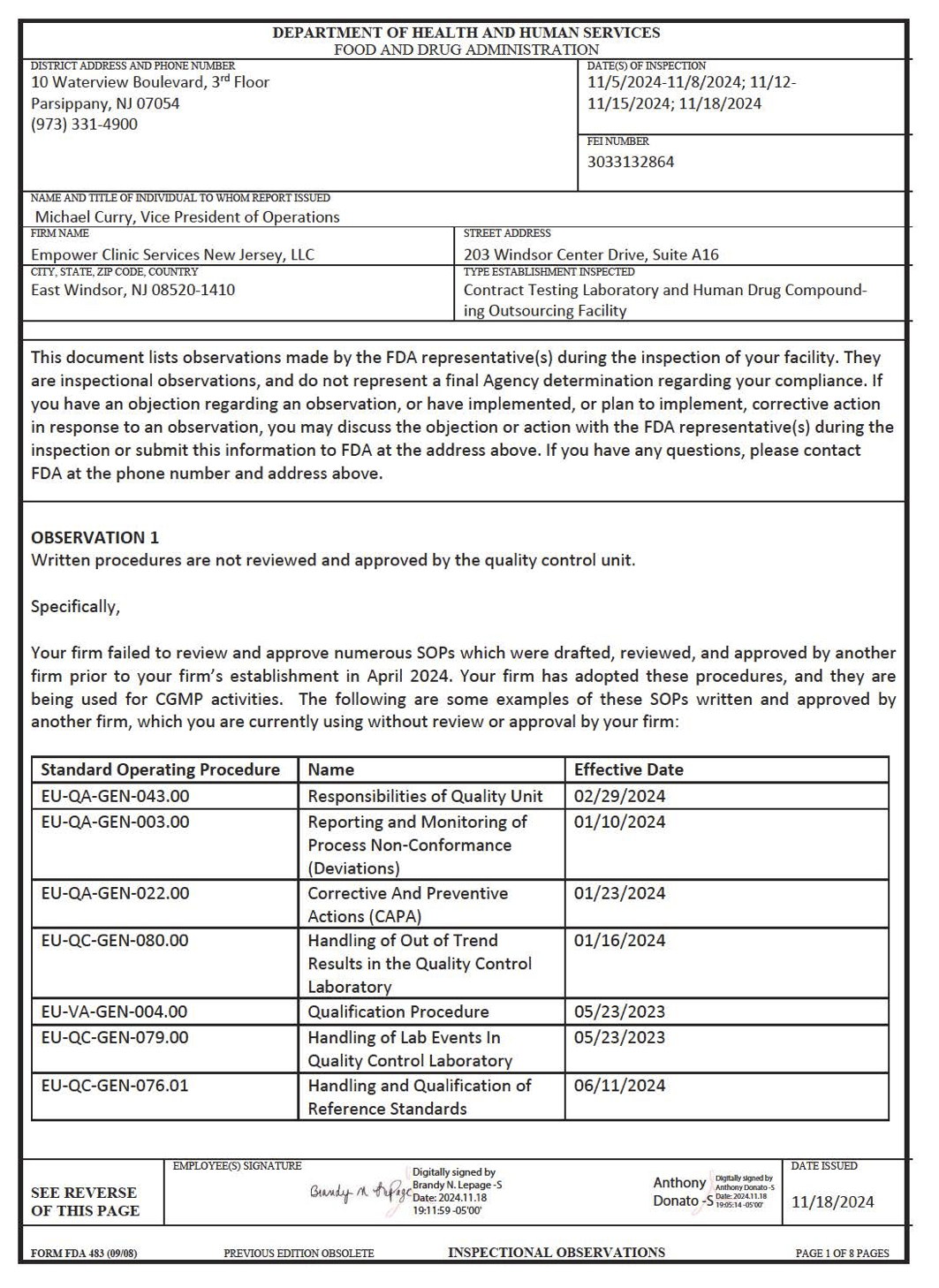
After several days of inspections in November 2024, the FDA issued a Form 483 to Empower Clinic Services highlighting violations related to drug manufacturing practices at its East Windsor, New Jersey facility. According to the report, the firm did not review or approve key standard operating procedures through its own quality unit. There were no documented performance qualifications for multiple critical testing instruments, and laboratory controls lacked scientifically validated test methods. Several tests required for stability and sterility assessments, including bacterial endotoxin testing, were not performed, despite being cited in protocols.

Read FDA's inspection observations about the New Jersey facility.
The inspection also noted significant microbial contamination. FDA investigators documented 49 pathogenic bacterial colonies in one water system and 401 in another, including strains such as Burkholderia cepacia and Ralstonia insidiosa. Burkholderia cepacia is challenging to treat, as it is resistant to many different kinds of antibiotics. Ralstonia Insidiosa can grow and survive in any kind of water, which is troubling given that the company that makes sterile injectables that are dispensed in water.
The report indicated that the facility’s quality unit had not undertaken thorough investigations or corrective actions in response to these findings. Additional concerns included inadequate controls over computerized systems and failures to record or justify deviations from established procedures or properly review critical system alarms.
As advocates for medicine safety, PSM is concerned about these inspection observations, especially in light of recent press coverage about Empower's Texas facility.
Learn about our concerns with compounded GLP-1s.