September 2, 2025: U.S. compounding restrictions have stemmed a flood of compounded semaglutide and tirzepatide
Major Stories
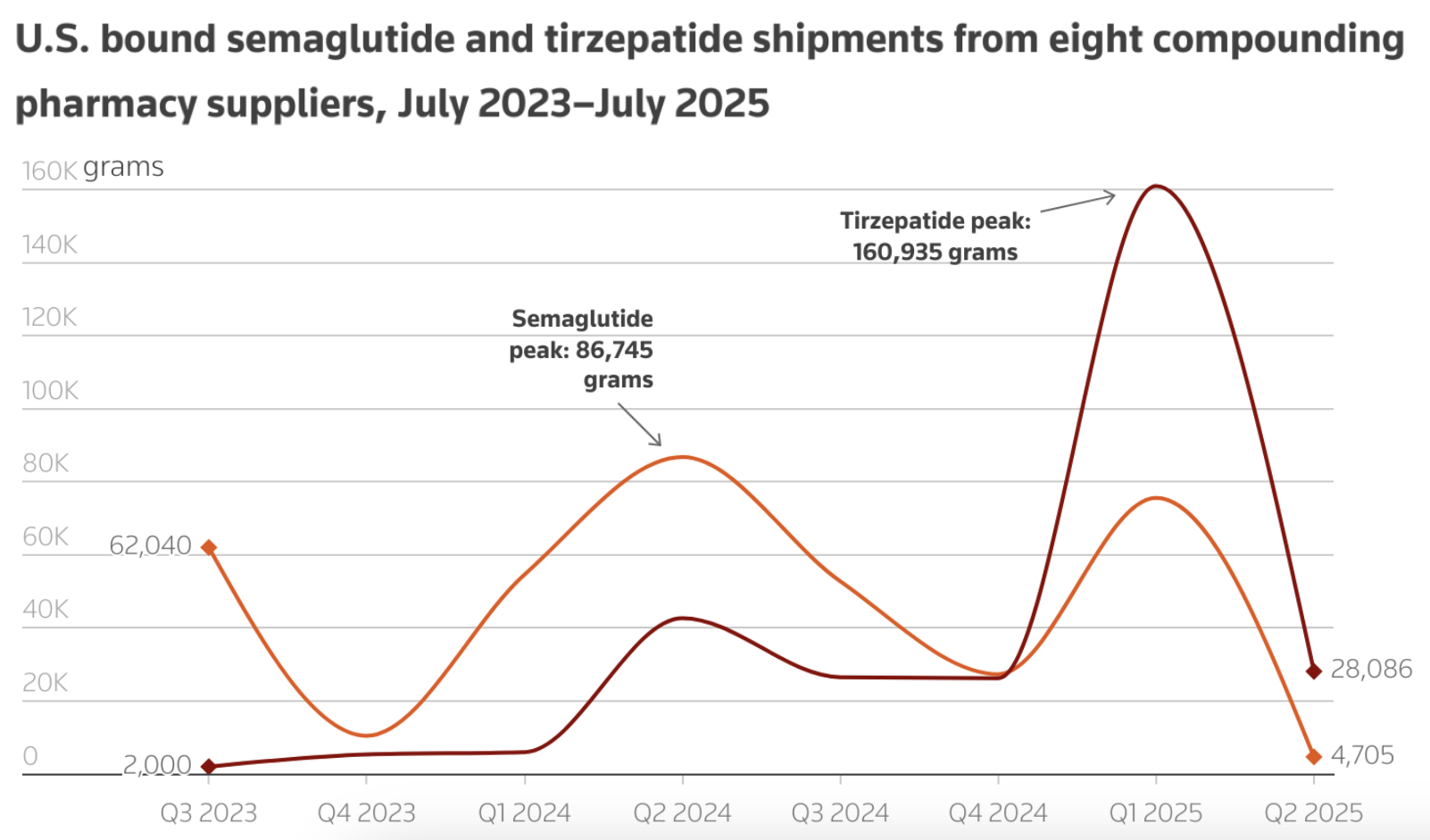
Chinese companies that supplied U.S. compounders with what Reuters called “makeshift” ingredients for billions of doses of weight loss injectables in 2024 are making generic semaglutide for markets where Novo Nordisk’s main patent is expiring in 2026. The change comes after the U.S. Food and Drug Administration (FDA) declared an end to semaglutide and tirzepatide shortages and restricted the sale of compounded versions of the drugs. 2025 FDA shipping data shows that shipments of semaglutide and tirzepatide dropped by 90 and 34 percent, respectively, from June 2024 to June 2025.
Source: Reuters sharing data from FDA's ITACS database
Domestic News
Undeclared tadalafil found in a supplement. A California pharmacist spoke out about PBM reform. CBP reminds Americans that smuggling prescription sedatives isn’t legal.
A supplement company announced that counterfeit versions of its male performance supplement, Green Lumber, contained undeclared tadalafil
Pharmacist Sonia Frausto’s guest commentary in Cal Matters described how pharmacy benefit managers’ predatory reimbursement practices led her to close her independent pharmacy in Sacramento, California. Frausto urged Governor Newsom and the California legislature to support Senate Bill 41, which would prevent PBMs from discriminating against independent pharmacies.
Intercepting zopiclone and zlprazolam from Colombia and Panama from travelers at Baltimore Washington International Thurgood Marshall Airport led U.S. Customs and Border Protection to remind Americans that “importing hundreds of prescription pills in traveler baggage and without a prescription is kind of a no-no.”
Agents with the Drug Enforcement Administration seized over 40 pounds of illicit drugs and an industrial pill press in a Manhattan, New York apartment building. Pill presses were also seized in connection with investigations in Washington, DC, and South Haven, Michigan.
In an August 29 editorial in The Tennessean, retired Williamson County Sheriff Dusty Rhoades identified illegally compounded weight loss medications as a major threat to public safety.
On August 28, PSM Executive Director Shabbir Safdar presented a webinar about the threats of counterfeit GLP-1s for the National Association of Boards of Pharmacy. The hour-long event was eligible for continuing pharmacy education credit. Review the course materials on NABP's site.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
Patient safety issues in the GLP-1 space this week
The U.K.'s Manchester Evening News reported on marketers promoting retatrutide to millions of users on social media despite the fact that the drug has not been approved anywhere in the world and cannot be obtained safely outside of Eli Lilly’s phase three clinical trials.
PSM was glad to see this Reddit poster seriously considering the source of their GLP-1 injections. It is a good idea to learn where your compounded medicine was manufactured and to check whether the facility has the appropriate licenses and is in good standing with regulators.
International News
London police seized counterfeit GLP-1s. Uzbek law enforcement shut down a large medicine-counterfeiting operation. Fake and substandard medicines reported in India, Pakistan, and Nigeria.
City of London Police seized £32,000 ($43,000) worth of suspected counterfeit Mounjaro, Ozempic and retatrutide from a Lancashire, U.K.-based fulfillment company that allegedly shipped the drugs to buyers without any medical oversight.
Authorities in Switzerland and Belgium warned about an uptick in counterfeit weight loss injectables.
Police in Uttarakhand, India arrested four pharmaceutical executives for their alleged involvement in a counterfeit medicine operation that distributed 1.8 million fake tablets bearing the packaging of prominent pharmaceutical companies between 2022 and 2025. The country’s Central Drugs Standard Control Organisation continues to report counterfeit and substandard medicines, among them antibiotics and cancer treatments, in circulation.
The Drug Regulatory Authority of Pakistan warned residents about counterfeit medicines, including antibiotics and pain relievers, being sold in parts of Punjab and Gilgit-Baltistan.
Uzbek authorities seized 11 pharmaceutical machines, 21 tablet molds, 389 kilograms of powders and tablets, and other items when they busted a fake medicine factory in Tashkent. The operation was producing counterfeit versions of a renal and a neurological drug, as well as an anti-inflammatory.
Nigeria’s medicines regulator issued alerts about counterfeit Postinor-2, an emergency contraceptive and Oxytocin, an injection that induces labor, controls postpartum bleeding, and supports lactation.

![August-27-2025-Reddit-compounded-Tirz Screenshot of a post that reads: Hi everyone, I was given compounded tirzepatide through a clinic here in Florida. The clinic provided prefilled syringes (wrapped in a napkin mind you). I called the next day to ask what brand it is but they told me it’s compounded. They didn’t disclose that but silly me didn’t ask before I agreed. I asked for the info and they sent me the paperwork showing testing from a lab in Michigan, and they told me the product is compounded by a “Texas 503A pharmacy.” When I pressed for details, the name I was given was [redacted] Here is the issue: the Texas Board of Pharmacy record for [redacted] shows they do not have sterile compounding authorization. Since tirzepatide is an injectable, I do not understand how this pharmacy could be the one preparing it. When I asked directly which pharmacy actually compounds the sterile tirzepatide, I was just told “we’re 503A.” I am now worried I do not know who really compounded this medication. Has anyone else run into this with a clinic? How do you verify the real licensed pharmacy behind these compounded tirzepatide shots? Any advice or shared experiences would be really helpful.](https://www.safemedicines.org/wp-content/uploads/2025/09/August-27-2025-Reddit-compounded-Tirz.png)
