September 15, 2025: Telehealth company must stop implying its compounded GLP-1 is FDA-approved, warns FDA
Major Stories
The New York Times reported that the U.S. Food and Drug Administration (FDA) warned telehealth company Hims & Hers to stop using language falsely implying that its compounded semaglutide is an FDA-approved product. The letter, which has not yet been released to the public, was one of about 100 sent as part of a crackdown on deceptive direct-to-consumer drug advertisements the agency launched on September 9.
A website pictured in a Novo Nordisk complaint, June 2023.
Domestic News
Ohio will tighten compounding regulations. Pennsylvania’s legislature met about pharmacy closures. Truthrx.org will host a summit on pharmacy benefit manager reform on September 24.
The Ohio’s Board of Pharmacy announced that new regulations will limit compounding pharmacies to making 250 units of a drug at a time. The limit, which includes sterile injectables such as GLP-1 weight-loss drugs, is meant to ensure that compounders are making medicines that are patient-specific now that shortages of semaglutide and tirzepatide have been resolved.
Two Massachusetts residents pleaded guilty to drug charges after investigators found a pill press, several kilograms of pills and fentanyl-laced powder during the July 2023 search of an apartment in Lynn.
A federal judge in eastern Washington sentenced Timothy Gary Maddox, of Spokane, Washington to 20 years in prison for running a counterfeit fentanyl pill operation busted in November 2023.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
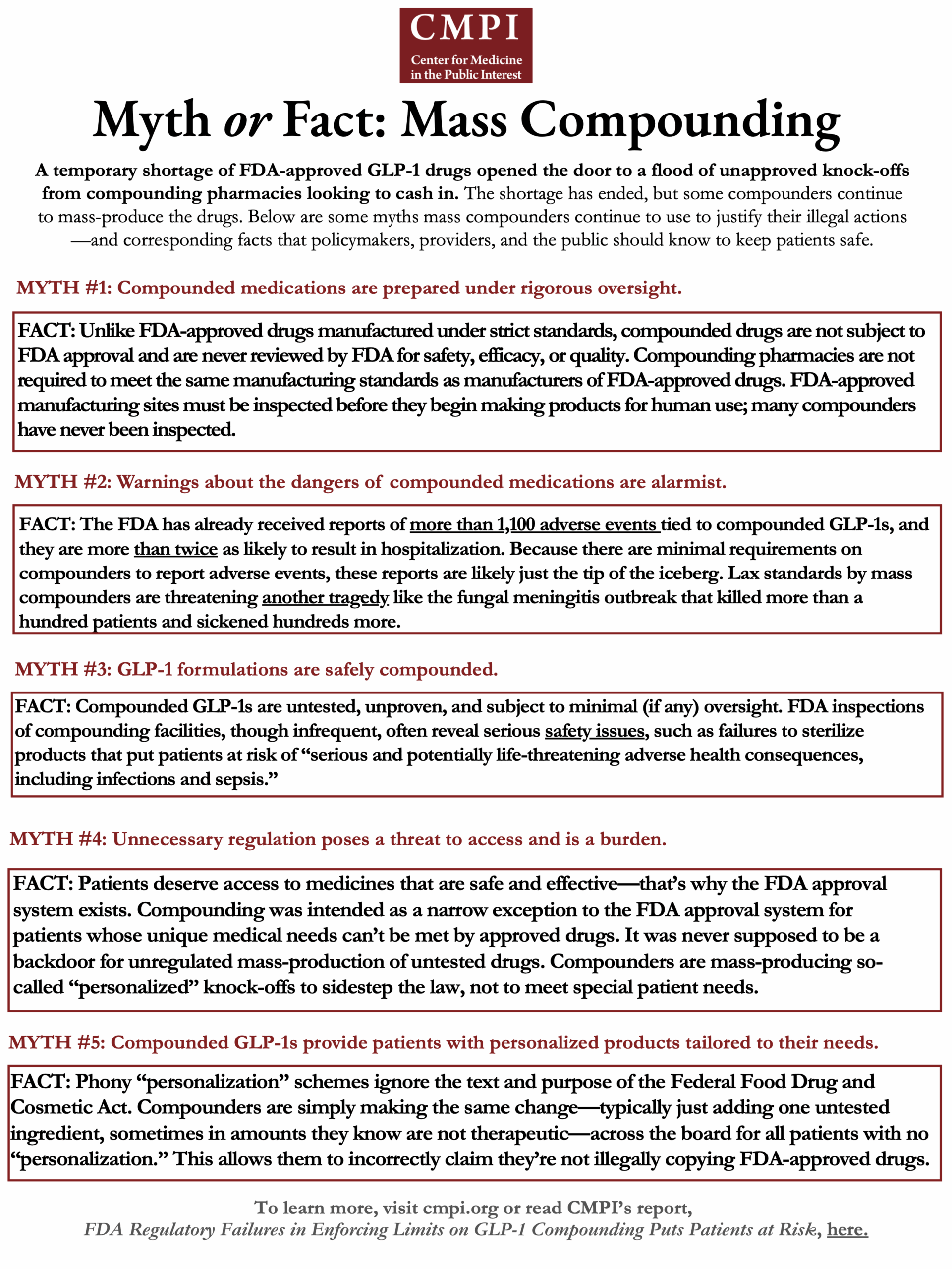
Review the Center for Medicine in the Public Interest's handout to dispel myths about mass compounding.
Pharmacy benefit managers (PBMs)
Advocates for pharmacies met Pennsylvania lawmakers at an informational hearing last week to discuss financial pressures, including PBM practices, that have led to the closure of 600 pharmacies over the last two years. The state's Department of Human Services secretary said the agency is studying the possibility of switching to a single PBM as Ohio did in 2019.
Register now for "Breaking the Status Quo," a virtual summit about PBM reform hosted by Pharmacists United for Truth and Transparency
Wednesday, September 24 at 10:00 am EDT
International News
Reports of fentanyl pills in Costa Rica and fake pharmacies in the Dominican Republic. Antimalarials seized in Nigeria.
The Latin Times reported on the impact of fentanyl and fentanyl pills in Latin America, noting that Costa Rican authorities had seen a sharp jump in pill seizures and are investigating suspected local fentanyl pill operations and that fentanyl deaths in Puerto Rico exceeded homicide rates between 2022 and 2024. The piece also mentioned that U.S. analysts had identified pharmacy websites shipping counterfeit pills from the Dominican Republic, as well as about $1.4 billion in transactions linked to fentanyl activity.
Nigeria’s National Agency for Food and Drug Administration and Control found 277 cartons (1,800 doses) of fake antimalarial drugs in a 40-foot container from China.