Gilead Sciences v Meritain Health, et al
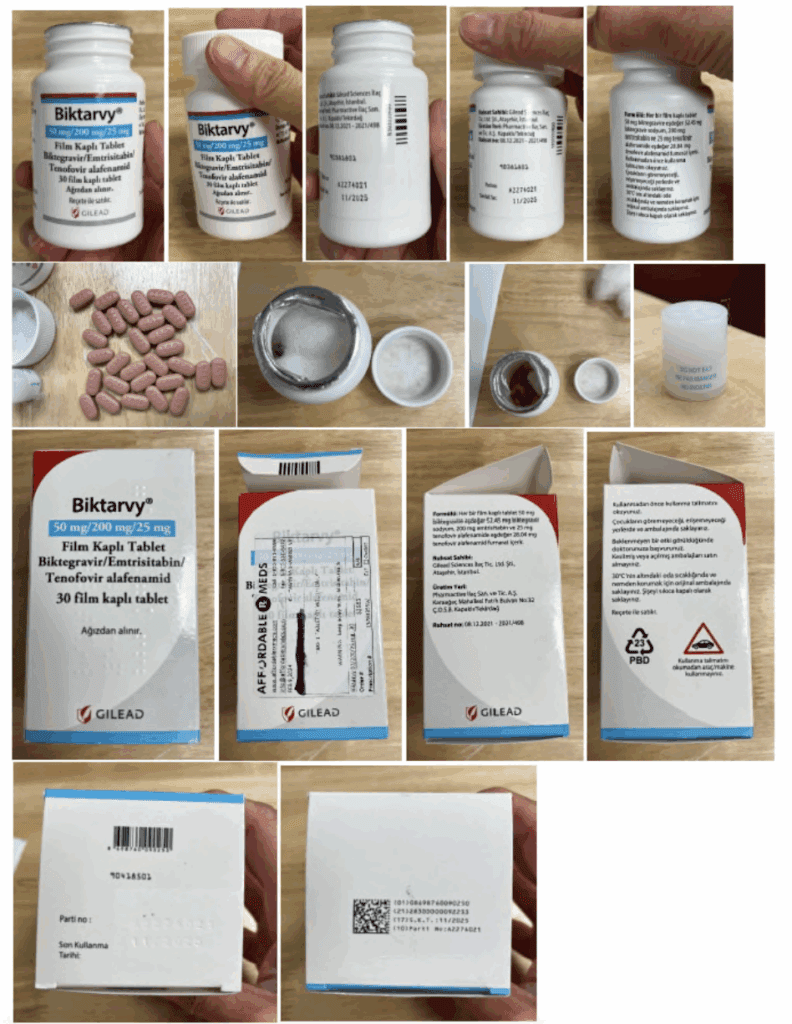
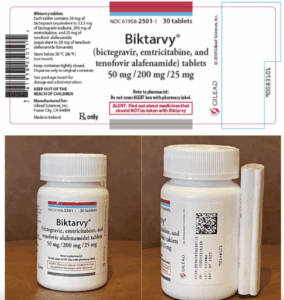
Gilead Sciences filed a complaint in December 2024 asking for a preliminary injunction to stop a network of companies they alleged were supplying patients with non-FDA approved, imported versions of Gilead's medicines via alternative funding programs (AFPs) to save their employer-sponsored health plans money. Gilead learned about the importation scheme after a patient who filled his HIV prescription with his health insurance received medicines mailed from a retail pharmacy in Turkey. The initial defendants in this case were:
- Meritain Health, a health benefits administrator;
- ProAct Inc., a pharmacy benefit manager;
- Gregory Santulli, the owner of Rx Valet, an AFP charged with finding the least expensive sources of medicine, and Advanced Pharmacy, a licensed U.S. pharmacy;
- Affordable Rx Meds, a broker that contracts with foreign pharmacies; and
- Fetih Eczanesi, a Turkish pharmacy.
In April 2025, a federal judge granted Gilead's request for a temporary injunction in an extensive opinion affirming that international sourcing of drugs through AFPs was not legal, and that companies and people facilitating this activity might be liable for it.
In September 2025 Gilead submitted an amended complaint naming CanaRx, ElectRx, ScriptSourcing, and several of their executives as defendants, alleging that they, too, had acted as alternative funding programs for Meritain Health and ProAct.
Court filings
December 2024:
- Complaint, Gilead Sciences v Meritain Health et al
April 2025:
- Memorandum opinion, Gilead Sciences v Meritain Health et al
September 2025:
- First amended complaint, Gilead Sciences v Meritain Health et al
- Memorandum of law in support of plaintiffs’ motion fortemporary restraining order, expedited discovery order, and order to show cause for a preliminary injunction, Gilead Sciences v Meritain Health et al