October 15, 2025: A JAMA study promoted med spa regulation; Hului's new documentary explores fake GLP-1s
Major Stories
A study says IV hydration spas need more oversight. Louisiana residents were hospitalized after receiving black market botulinum toxin injections. A new Hulu documentary delved into the counterfeit GLP-1 market and Fierce Healthcare covered AFPs.
A new study highlights major gaps in the regulation of IV hydration spas, an increasingly popular sector of the U.S. medical spa industry. Journal of the American Medical Association (JAMA) Internal Medicine published a review of state policies, med spa websites, and secret-shipper calls, which found that nearly all med spas made health claims about IV therapy benefits, but rarely cited evidence, and few required medical consultations or disclosed risks.
Because these spas operate as state-regulated compounding pharmacies rather than FDA-supervised manufacturers, oversight remains limited. Learn more about the risks of med spas and policies to address them in our med spa handout.
Two Louisiana residents were hospitalized with severe botulism symptoms after receiving botulinum toxin injections from unlicensed sources, prompting a warning from the Louisiana Department of Health. One purchased the toxin online; the other was treated by an unqualified provider in a non-medical setting.
A recent Hulu documentary exposes the growing black market for fake GLP-1 drugs. It features interviews with medical experts, federal investigators, pharmaceutical security officials, and public figures like Aisleyne Horgan-Wallace and Dolores Catania, offering an inside look at the risks behind the counterfeit drug trade.
Fierce Healthcare wrote about alternative funding programs (AFPs), which aim to cut specialty pharmacy costs for employers who self-fund their insurance plans by diverting high cost prescriptions to assistance programs or international sourcing. According to the article, they haven’t consistently delivered savings and they have caused harmful delays that increased patients’ ER or urgent care visits, prescriptions, and disease progression. PSM has shared our concerns about AFPs here.
Domestic News
PSM’s new freight fraud report offers a view of commercial-scale drug imports. Sheriffs across the country warned about the dangers of counterfeit weight loss medicines.
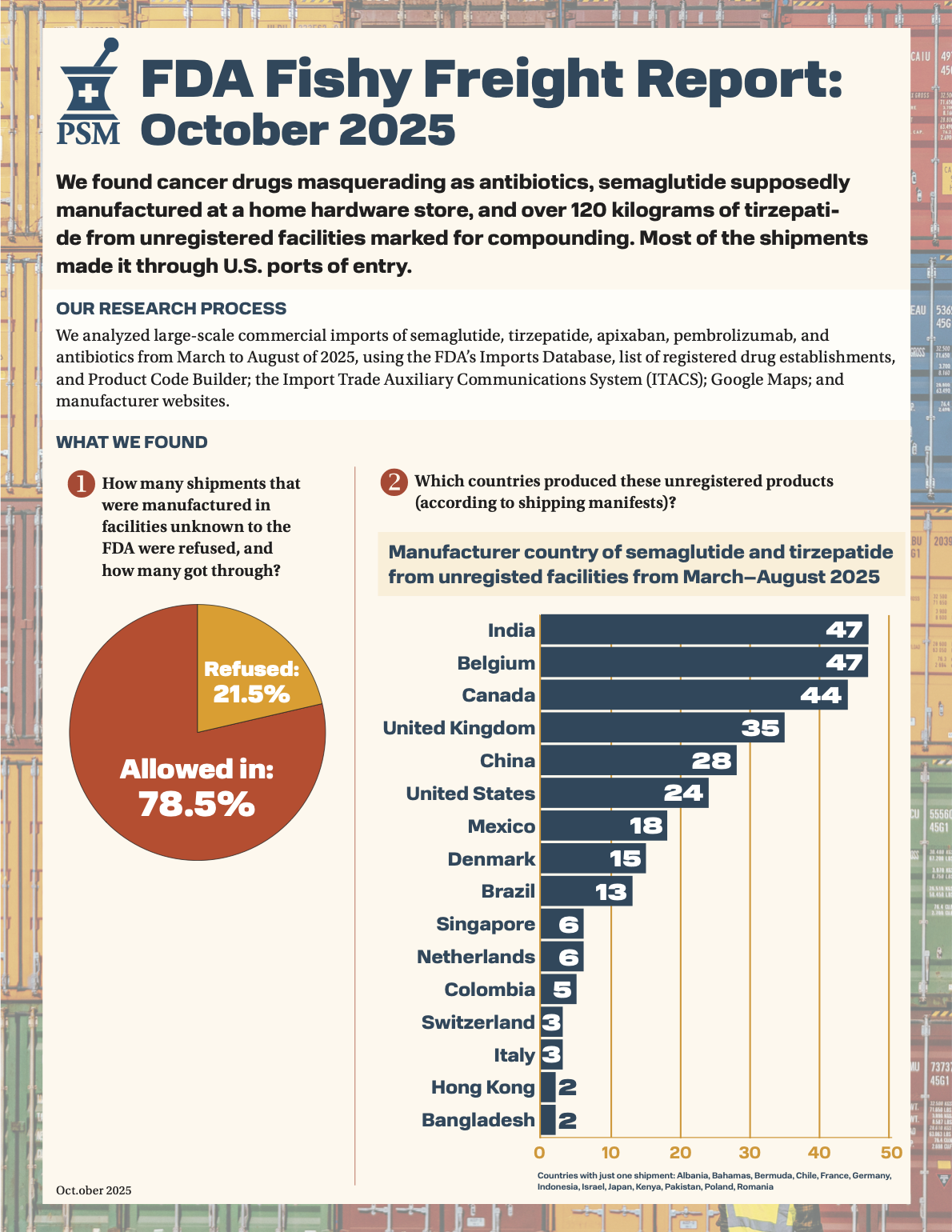
PSM’s six-month freight fraud report is out. U.S. Food and Drug Administration (FDA) import data showed cancer drugs masquerading as antibiotics, semaglutide from a home hardware store, and over 120 kilograms of tirzepatide from unregistered facilities marked for compounding. Read it here.
Op-eds by current and former sheriffs in Chilton County, Alabama, Citrus County, Florida, Warren County, Kentucky, Livingston County, Michigan, and Williamson County, Tennessee warned residents about counterfeit and illegally compounded versions of Ozempic and similar medications making their way into the country.
Read October's Fishy Freight Report.
Regulators protecting patients in the news
The FDA's Office of Compounding Quality and Compliance urged state medical boards to remind practitioners to prioritize comprehensive, continuous care and avoid practices that compromise patient safety, as superficial relationships that solely facilitate prescriptions, particularly through third-party telehealth platforms or online sellers, risk fragmenting care, obscuring the quality or source of drugs, and leaving patients without proper follow-up or risk education.
The FDA issued warning letters to several companies for CGMP violations related to over-the-counter products. The FDA cited the California and Texas companies for failing to adequately test for diethylene glycol and ethylene glycol and for sterility and bacterial issues. The agency rebuked the California company and a Georgia facility for violating FDA regulations on unapproved and misbranded products.
The Ohio Board of Pharmacy issued eight more suspensions of clinics for serious violations involving unapproved, adulterated, or improperly sourced drugs, including compounded GLP-1s, neurotoxins, and other injectable products. Med spas and dentists across the state were cited for issues including unlicensed compounding, improper labeling, expired or foreign-sourced drugs, lack of refrigeration records, and failure to maintain prescriber oversight. To learn more about why Ohio is leading the country in med spa safety regulation, read our med spa handout.
Read about the need for better med spa regulation.
Pill presses
A Blytheville, Arkansas man was arrested after police executed three search warrants and seized over 100 grams of ecstasy, 11 grams of fentanyl, 90 pills, two vehicles, and a pill press.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
International News
The United Kingdom’s health regulator warned about unapproved weight loss pills. Fake cancer drugs were reported in Kenya and India.
U.K. health authorities warned consumers about a surge in online sales of “Ozempic-like” GLP-1 weight loss pills that contain orfarglipron. The drug is still in clinical trials and is often mislabeled as “for research only” to evade legal penalties. Authorities emphasized that purchasing unlicensed medicines is illegal and advised consumers to avoid online sources, seek guidance from healthcare professionals, and use only regulated, approved medications.
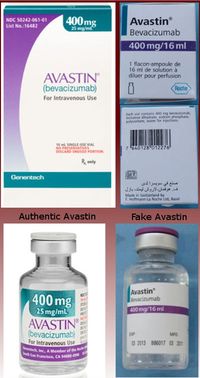
Read about fake Avastin in the U.S. (Image: FDA)
A Guardian investigation found that TikTok influencers and Telegram sellers are fueling a black market for retatrutide, another unlicensed weight-loss drug that is still in clinical trials. Influencers direct followers to WhatsApp for purchases, while Telegram groups share injection guides and testimonials. Experts warn that these unregulated products are dangerous, as buyers cannot verify contents, dosage, or sterility.
Kenya’s Pharmacy and Poisons Board issued an urgent warning about a counterfeit batch of Avastin (bevacizumab), a critical cancer treatment. The U.S. also has a history with counterfeit Avastin.
Eight men in Delhi, India were arrested for running a network selling fake or unauthorized cancer drugs sourced from countries, including Egypt and Turkey. Seized medications included high-value treatments like Opdivo, Pembrolizumab, Cetuximab, and Lenvatinib, along with other critical injections and capsules.