Prescription Drug Affordability Board Activity, September 2025
Activities Summary
Colorado: Colorado's next meeting will be October 3, 2025.
Maryland: The PDAB met on September 29, 2025 to hear public comment about UPL-setting for Jardiance and Farxiga and to consider policy approaches to lower the costs of these drugs for Maryland patients.
Oregon: The PDAB met on September 17, 2025 to discuss potential policy recommendations for its 2025 annual legislative report, to consider how to deal with volunteered trade secrets, and to hear high-level information about Jardiance, Mounjaro, Ozempic, Rybelsus and Trulicity. The next meeting is October 15, 2025.
Washington: The next PDAB meeting will be on November 19, 2025.
Activity by State
Maryland
Agenda | Meeting Recording | Policy Review Process and Preliminary Recommendations Presentation (Updated and Revised September 24, 2025) | Written Comment Packet
Call meeting to order and proclamation - Dr. Joseph Levy
The board read a proclamation thanking Dr. Levy for his insights and service to the PDAB during his term. There was also an announcement of the new appointment of Dr. Georges C. Benjamin.
Public comment
Reuben Collins, President of the Charles County Board of Commissioners, thanked the board for their work and talked about how UPLs would ease burdens for the county's taxpayers.
Marc Elrich, Montgomery County Executive, spoke about Maryland as a leader in the country, and also urged the Board to pass the UPL quickly.
Steuart Pittman, Anne Arundel County Executive, reiterated his thanks for the work the board has done and urged the board to pass the UPL.
Calvin Ball, Howard County Executive, also thanked the board for their efforts to lower costs for residents. He urged the board to set UPLs on Jardiance and Farxiga.
Derek Flowers from the Value of Care Coalition talked about threats to patient access that UPLs present. He urged the board to look at policies that address transparency of benefit design and cost sharing at the counter, which would ensure patients received meaningful relief without sacrificing access.
George Huntley, from the Diabetes Patient Advocacy Coalition, testified about concerns among patients about access should a UPL be passed.
Tiffany Westrich-Robertson, from Ensuring Access Through Collaborative Health and the Patient Inclusion Council, said that the Board was not acknowledging that Jardiance and Farxiga have no true therapeutic alternatives, and that they were the only drugs that treat such a broad range of issues. She also mentioned that she heard in the informational hearing that the PDAB has a supplemental rebate structure solution that would address concerns related to the UPL action plan, but she requested more information before weighing in on this.
Policy review process - cost drivers for Farxiga and Jardiance
Staff discussed the steps the board had already undertaken to get to this point in the affordability review process. They mentioned that there a draft of the preliminary determination report developed from an informational hearing, stakeholder council meetings, staff research, and data from eligible governmental entities, would be available for public scrutiny in the coming months.
Feedback from the informational hearing
Staff summarized comments from the informational hearing, which covered:
- the struggles of senior citizens and lack of patient engagement.
- personal experiences with the inability to afford medications and support for UPLs,
- the life-changing impact of these drugs and concerns about patient access if a UPL is set,
- plan design issues,
- surveys showing that insurance barriers have the most impact costs and
- the promotion of PBM reform rather than a UPL to control costs.
Stakeholder Council Meeting feedback
The Stakeholder Council Meeting yielded similar comments from the public that can be used in the policy review process. It was organized by tying comments to four circumstances that create affordability challenges as identified by the board, and summarizing conversations related to each.
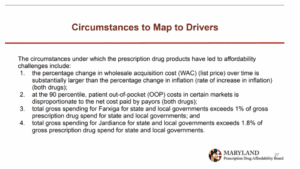
Circumstance 1 related to the percent change in WAC being substantially larger than the rate of inflation. The council highlighted that benefit design and PBM practices were contributing to the increase in WAC, and that UPLs had been successfully implemented in other countries.
Circumstance 2 was that at the 90th percentile, the patient out-of-pocket cost in certain markets was disproportionate to the net cost paid by payors. The council found that insurers using accumulator programs and benefit design that shifted cost to the patients were drivers of this situation. A policy solution could be to support legislation to end this practice, which the industry would support.
Circumstances 3 and 4 involved total gross spend for state and local governments exceeding 1% of gross prescription drug spend for state and local governments for Farxiga, and 1.8% for Jardiance. The council found that the driver for this was a high utilization rate, which is natural for a state like Maryland, with high diabetes rates. The stakeholder council also cited insurance benefit design (copays, coinsurance, and deductibles) as a driver for affordability challenges.
Staff Policy Research
Staff also researched policies implemented by other entities and jurisdictions to contribute to information gathering for the review process. They collected information from eligible government entities, including utilization, spending, costs, benefit design, formulary placement, rebates, discounts, price concessions, and other relevant information. This material is primarily confidential.
According to the statute, each policy recommendation must address the drivers of the four circumstances that the board identified as creating affordability challenges for Jardiance and Farxiga. The staff identified at least one driver for each circumstance:
- Manufacturer and PBM negotiations for drug rebates (circumstance 1);
- Manufacturers prioritizing profit by increasing the WAC (circumstance 1);
- Insurance companies select preferred drugs based on rebates (circumstance 2);
- High deductible health plans, high coinsurance rates (circumstance 2); and
- High utilization rates (circumstances 3 & 4)
Policy review process: non-UPL policy recommendations: Farxiga and Jardiance
Staff presented preliminary policy recommendations to the board, starting with non-UPL policy recommendations that the board would either vote to direct staff to continue to research or not to prioritize going forward. They began by listing policies suggested by the public and stakeholders that were not being presented to the board because of existing legislation or the failure of the policy to address an identified driver. These included copay caps, protections for utilization management, and PBM reform.
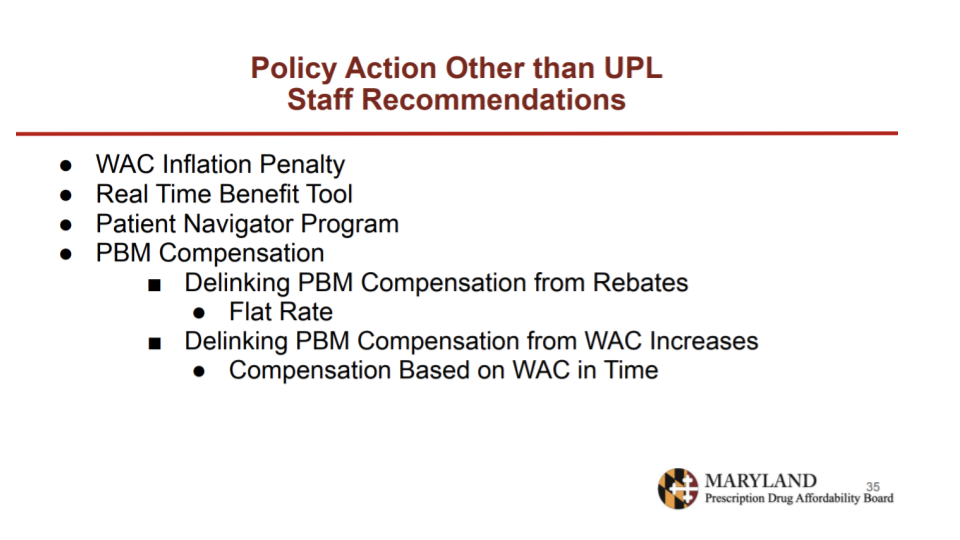
The first policy staff presented was a WAC inflation penalty, which would require a manufacturer to pay a penalty on the gross Maryland revenue attributable to the increase in WAC above inflation. This policy has been implemented in other states, though there is no clear understanding of whether it is effective. The board seemed hesitant to pursue this policy recommendation, citing a lack of evidence about its efficacy.
The second policy staff proposed was to require plans to adopt benefits software that would allow patients and prescribers to access real-time formulary and benefit information to shop for lower-cost alternative therapies. This was already a requirement for Medicare plans under the Inflation Reduction Act, so staff believed it would be easy to implement. There is, however, no conclusive evidence about the outcomes of these tools. The board raised concerns about ERISA, the complexity of this system for small plans and hospitals; enforcement, prescriber burden, and it addressed any drivers.
A third policy proposal was to establish a program to help patients search for patient assistance programs and help them apply using an existing technology vendor. This was largely based on the Kentucky Prescription Assistance Program. Staff found that this approach yielded a dramatic return on investment in other states. The board raised concerns about feasibility, whether start-up costs for the program would be worth it, and whether it would be an effective tool to lower drug costs for a broad majority of the population.
Chair Mitchell voiced frustration that the board has accomplished “basically nothing” in the past five years. He said that no policy recommendation will be perfect; that the return on investment of the board has been terrible, and they need to pass something that helps people, UPL or not.
The last non-UPL policy recommendation involved two approaches to changing PBM compensation. The first would be to disconnect PBM compensation from rebates, setting compensation at a negotiated flat rate instead. The second would be to disconnect PBM compensation from WAC increases, which would eliminate incentives to increase the list price to get a larger rebate. Staff recounted stakeholder concerns that these approaches could increase net costs by removing the incentive for PBMs to negotiate aggressively, or that they could encourage higher launch prices if reimbursements are based on fixed WACs.
The board was most supportive of moving forward with recommending the WAC inflation penalty, the patient navigator program, and PBM compensation legislation.
Policy review process: next steps in UPL policy: Farxiga and Jardiance
The board moved on to discuss next steps in the UPL policy. Staff said that the strength of UPLs are that they protect state and local government entities from WAC increases. Weaknesses include that:
- the mechanism of UPLs is directly tied to manufacturer decisions to increase WAC prices
- the impact of the savings to the government is based on the net price that the entity is currently paying and the UPL amount, and
- Farxiga may have generic competition as early as April 4, 2026
The next steps in the process would be for the staff to publish a framework for how this policy would be implemented. The board decided that staff should continue to gather information about UPL setting.
Administrative update
Staff gave a staffing update, stating that they were sending offer letters for a quantitative data analyst. They were also planning on posting an administrative specialist job for applications.
Staff will also publish a draft report of the cost review study and the UPL methodology for public comments.
The next board meeting will be on November 17, 2025.
Oregon
Oregon PDAB Meeting, September 17, 2025
Program update
The senior policy analyst presented PDAB program updates announcing that Sarah Young had been named as the next Executive Director of the PDAB and the Drug Price Transparency Program. She will begin her role on October 6. A new board member, Michele Koder, was also selected, and awaits a Senate confirmation hearing at the end of the month. Her first meeting with the board will be on October 15.
Three board members were also reappointed: Dr. Dan Hartung, Dr. Chris Laman, and Dan Kennedy.
Lastly, staff talked about the 9th Circuit Court ruling in PhRMA v. Stolfi, which upheld Oregon's HB4005, the drug price transparency law. HB4005 requires drug manufacturers to report information related to certain drugs Oregon Department of Business and Services Prescription Drug Price Transparency Program.
General public comment
Primo Castro, from the Biotechnology Innovation Organization, expressed concern about patient access and said that the board's mission was flawed. He was also concerned about a lack of scientific rigor associated with the drug scoring framework, which has not been vetted publicly, and he said was subjective.
Lauren Sandt from the Caring Ambassadors Program spoke about the fact that trade secrets and confidential information were on the agenda for the meeting. She asked whether they were going to update the cost information and if the board would have time to review changes before making decisions on the drug being reviewed. Lastly, she asked the board to consider two policy recommendations: allowing PDAB members to interact with public testimony, and the establishment of a stakeholder advisory board.
Jessica McBride from the Oregon Coalition for Affordable Prescriptions talked about how Oregonians are struggling because of the costs of drugs, and about factors that contributed to the high cost of drugs. She expressed appreciation that pharmacy benefit managers were included in the policy recommendations.
Dahria McGrew, on behalf of PhRMA, said they were glad that the board’s policy recommendations addressed problems across the supply chain that are driving up costs. She reiterated concerns about the scoring framework being arbitrary and subjective. She renewed her request for a data dictionary for the review packets.
Dr. LuGina Mendez-Harper, a pharmacist with pharmacy benefit manager Prime Therapeutics, testified about the board’s mission to protect stakeholders from the high cost of prescription drugs, and to provide recommendations to the legislature to make drugs more affordable. She urged the board to keep the mission in mind when making policy recommendations.
Vanessa Lathan from the Patient Inclusion Council urged the board to slow down the drug review process and consider how the process could hurt patients. She expressed concerns about medical switching and reduced access. She also said that patient and provider voices should be central during this process.
Derek Flowers from the Value of Care Coalition brought up past concerns about UPLs and says he is supportive of the policy recommendations. He also mentioned a lack of patient and provider voices, and urged the board to consider drugs on an individual basis, not in relation to each other.
Volunteered trade secret information
In August, the board received a public comment letter related to trade secret information provided by a manufacturer, which the board does not yet have a policy or process to deal with. A staff member discussed options that the board could adopt and processes that other PDABs use to get feedback. There was further discussion about next steps.
According to Oregon's statute, the board may meet in executive session to discuss confidential information, but—unlike other state PDABs—news media is allowed to attend these executive sessions. The board needs to establish a process that will ensure continued confidentiality, given the media’s access to these conversations.
One board member raised concerns about the kind of transparency the board could expect from manufacturers, given that the media could hear or see the information. Other board members echoed this concern, and they eventually asked if this information would be necessary for their decision-making. Members also raised concerns about defining “media,” the risks to board when discussing information in front of the media, and the time it would take to make this policy.
A board member pitched the idea that media should register or sign a confidentiality notice, a proposal that other board members seemed to generally support. Another board member asked if this rule could be changed by the legislature, which staff said they would look into. The board also asked whether they could make it their policy not to accept trade secret information, and present this to the legislature in their policy recommendation. Staff also agreed to look into that.
Chair Bailey said she was uncomfortable discussing trade secrets with the media present, mentioning that the previous chair cited this issue as one of the reasons he left. Another board member echoed her. The board did not come to a clear conclusion, and they will revisit this issue at the next meeting.
Policy recommendations for the 2025 annual legislative report
The board discussed the concepts that each board member submitted to the staff, including potential recommendations for instituting one PBM for all Medicaid and managed care patients, increasing transparency in the drug pricing process, and expanding the focus of the PDAB to discuss other avenues to improve affordability. Most board members supported all the ideas.
Of note, Vice Chair Amy Burns disagreed with the idea of expanding the PDAB, supporting the creation of new boards to deal with problems like PBMs or Medicaid policy instead. If the board’s focus did expand, Burns felt current membership was not well positioned to discuss other issues, and the people sitting on the board would need to change.
Methodology for drug reviews and scoring rubric, and worksheet
Staff told the board that they were going to stop collecting information and finalize packets with the edits the board made starting next month, so affordability decisions could be made. They also had updates to the rubric, including adding domains. Chair Bailey spoke about adding the rubric to all the packets, as she found it easier to follow.
Drug reviews: Jardiance, Mounjaro, Ozempic, Rybelsus, and Trulicity
Jardiance Drug Review
The board turned to the first antidiabetic for review, Jardiance. Staff talked the board through some of the high-level statistics to get the conversation started, including cost per enrollee, out-of-pocket costs, and health inequity information. Chair Bailey mentioned that this drug seemed to have a broader indication, which could be an advantage of this medication over some of the therapeutic alternatives. Dr. Burns explained that Farxiga has a similar profile to Jardiance.
Mounjaro Drug Review
As they were looking through the statistics associated with Mounjaro, Chair Bailey asked the pharmacists on the board to clarify a statistic about the cost per month of the dose, which can vary significantly based on the prescription. The board decided that this needed to be a nuanced comparison with other similar drugs.
Ozempic Drug Review
A board member asked how the increase in the price of Ozempic compared to inflation, and whether it was high enough that it needed to be reported to another board in the state.(There is legislation that requires this if there was more than a 10% increase, but this does not apply to Ozempic.) Board members also discussed the data related to average unit WAC. It didn’t make sense to the board members and staff will clarify it in future meetings.
Rybelsus Drug Review
Staff talked through high-level statistics for Rybelsus, and the board had no further comments.
Trulicity Drug Review
Staff talked through high-level statistics for Trulicity, and the board had no further comments.
Meeting conclusion
To end the meeting, Chair Bailey talked about out-of-pocket costs for Medicare recipients, noting that the board should keep the lack of coupons and assistance for that population in mind. Vice Chair Burns also mentioned that they add a policy recommendation to look at capping prices to the federal MFP. This triggered a discussion about the place of the board and whether this would be an effective recommendation. Vice Chair Burns stressed that the board should be lowering costs to patients as a support for her suggestion, and suggested that one of the pharmacists on the board was not looking at the issue holistically. The board said they will be making decisions about which drugs are unaffordable in January, after comparing all drugs in November and December.
The next meeting will be on October 15, 2025.