Article Topic
Methanol-Contaminated Rubbing Alcohol Recalled by FDA
This is a reprint of an FDA Alert. Essaar Inc. Issues Voluntary Nationwide Recall of Rubbing Alcohol Contaminated with Methanol When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. Company Name: Essaar Inc.…
[...]Welcome to 2021. Here’s the next 6 months in counterfeit medicine news.
If you’re a reporter who covers healthcare and especially public health issues, this is what we think you should be watching for in the next six months of a better 2021
[...]Counterfeit Medicine News for the Week of December 14, 2020
In PSM’s round-up this week: Make sure you’re getting the REAL COVID-19 vaccine, plus prosecutions and seizures involving dangerous supplements, a fake online pharmacy and a variety of counterfeit pills.
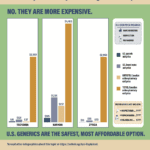
[...]Are safety-tested imported drugs still cheaper? No. They are more expensive.
Testing medicine for legitimacy is a complicated process. Across 24 different prescription medicines, the average cost to test a single dose is $2,750. However, ensuring that a batch of 100 pills is 90% certain to be safe requires testing at least 22 pills. Achieving 99.999% certainty requires even more testing, at tremendous expense. Once you’ve done the required testing, U.S. generics are cheaper. To learn more about this topic, read PSM’s summary: safedr.ug/Acri-Explained.
[...]Importation 101 for 2021
Many advocates and legislators are considering whether to pursue Canadian drug importation in 2021, but importation is not and never has been a viable option.
Watch our video to learn the basics and for an update on four states that have been trying to move forward with this idea.
[...]Counterfeit Medicine News for the Week of December 7, 2020
In PSM’s round-up this week: ongoing COVID-19-related fraud, Europol’s Operation Shield, and counterfeit pill news in six states.
[...]Canada’s Actions a Death-Knell for Drug Importation in Florida
This editorial by the Sun Sentinel Editorial Board was published in The Sun Sentinel on December 9, 2020. The Editorial Board consists of Editorial Page Editor Rosemary O’Hara, Dan Sweeney, Steve Bousquet and Editor-in-Chief Julie Anderson. There Go Those Cheaper Drugs. Remember Florida’s plan to save money by importing prescription drugs in bulk from Canada?…
[...]Counterfeit Medicine News for the Weeks of November 23 and 30, 2020
In PSM’s round-up this week: Canada banned exporting drugs to the U.S. if it will cause drug shortages at home, the FTC warns about fake COVID-19 testing centers, several large counterfeit pill busts, and more.
[...]Canadian writer applauds country’s effort to protect its drug supply
This editorial by Joyce Nelson appeared in Counter Punch on December 4, 2020. Nelson is a researcher and writer whose work appears in a wide range of magazines, newspapers and websites.
[...]Organizer explains why drug importation is dangerous and “doomed to fail”
This editorial, written by Earl D. Fowlkes Jr., appeared in the Washington Blade on December 4, 2020. Fowlkes is the president and CEO of the Center for Black Equity.
[...]