Article Topic
Colorado Department of Regulatory Agencies updates Joint Budget Committee on litigation costs
A March 12, 2025 memo said the state had paid more than $150,000 defending itself in a lawsuit Amgen had filed over plans to set an upper payment limit on its rheumatoid arthritis treatment, Enbrel.
[...]March 31, 2025: Senator asks how FDA will be accountable for unregulated active pharmaceutical ingredient (API) imports
Senator Jim Banks asked pointed questions about how FDA will stem the tide of semaglutide and tirzepatide coming from unknown facilities.
[...]Government Sentencing Memo, USA v Aaron Michael Thomas, February 24, 2025
United States District Court Northern District of Oklahoma USA v Aaron Michael Thomas Government Sentencing Memo Filed February 2025 Read the document.
[...]Prescription Drug Affordability Boards by the numbers
Prescription Drug Affordability Boards are an expensive idea that has not produced results. It’s time to move on.
[...]Handout: What is an Alternative Funding Program?
Some employers are hiring consultants, sometimes called “Alternative Funding Program (AFP) vendors” or
“importation program vendors,” to supply employees and their families with illegally imported and unsafe
medicines.
March 17, 2025: A Texas Pharmacist gets 17 years for $145m skin cream billing fraud
A Texas man billed government programs millions for creams compounded by untrained teenagers. News about counterfeit medicine in six U.S. states.
[...]March 10, 2025: Patients transitioning from compounded diabetes / weight loss injections: Stick to licensed sources
FDA’s shutdown of GLP-1 compounding could lead to a new era of risk for U.S. patients.
[...]FDA Alert: Update on Previous Nationwide Warning About Presence of Undeclared API in Capsules
The FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
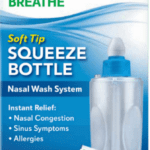
[...]FDA Alert: Recall Issued on Nasal Wash System Due to Microbial Contamination
An FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
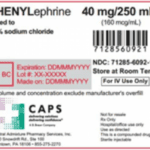
[...]FDA Alert: Recall Issued on Injectable Product Due to Potential Particulate Matter
An FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
[...]