Avoid #covidscams - A Partnership for Safe Medicines Public Education Campaign
Avoid #COVIDScams and Counterfeiters: Tips to Purchase Medicine Safely
Ask your pharmacist about how much medicine you should buy: hoarding can create unnecessary shortages.
If you are experiencing hardship contact NeedyMeds, RX Outreach and Medicine Assistance Tool to find patient assistance programs to help you afford your medicines.
If you are buying over the counter or prescription medicine online, buy from a .pharmacy pharmacy. Pharmacies in other countries are not safe, even if they "look safe" to you.
Download our guide, AVOID SCAMS & COUNTERFEITS: Quick Tips to Safely Purchase Medicines Online (in English and Spanish) to learn more, and our #covidscams bookmark, which is a quick reference about five kinds of online crime that have spiked since the coronavirus emerged.
For up to to the minute news, track our coverage about the five types of COVID-19 scams.
Adopt this campaign and help spread the word!
Post our one pager to your website and to social media. Use the hashtag #covidscams to help raise awareness of criminals using the crisis to prey on people. Drop us a note at editors@safemedicines.org to let us know you're helping! Click here for sample tweets.
Tell Congress to kick COVID-19 scammers off the Internet!
Learn more and write congress on our TAKE ACTION page.
#covidscam News
Use of these tests may cause serious adverse health consequences or death. There is also a risk of injury if users follow any labeling instructions directing self-collection of nasopharyngeal or oropharyngeal samples.
This is a reprint of an FDA Alert. Mesa Biotech, Inc., Recalls Certain Accula SARS-CoV-2 Tests for Risk of False Positives Caused by Contamination The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death. Recalled Product Product Name: Accula…
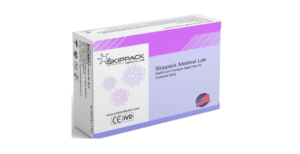
This is a reprint of an FDA Alert. Do Not Use Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test: FDA Safety Communication May 10, 2022 The U.S. Food and Drug Administration (FDA) is warning people not to use the Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test (Colloidal Gold). This test is not authorized, cleared, or approved…
This is a reprint of an FDA Alert. SD Biosensor Recalls STANDARD Q COVID-19 Ag Home Tests That Are Not Authorized, Cleared, or Approved by the FDA and May Give False Results The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious…
The U.S. Food and Drug Administration (FDA) is warning people not to use the SD Biosensor Inc. STANDARD Q COVID-19 Ag Home Test. The test is not authorized, cleared, or approved by the FDA for distribution or use in the United States. The FDA is concerned about the risk of false results when using this unauthorized test. This unauthorized test may be packaged in a white and magenta box.