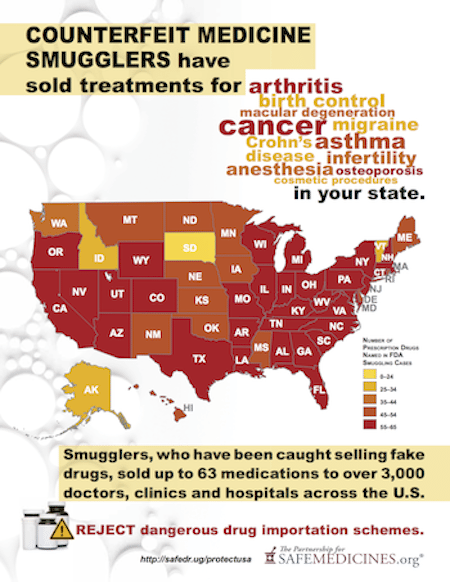
Bipartisan Safety Issues: How Many Different Kinds of Black Market Medicines have been Sold in Your State?
In 2012, the FDA discovered that American doctors had purchased illegally imported, counterfeit Avastin from subsidiaries of CanadaDrugs.com. They saved money on the medicine for their patients, but their sources were unreliable: some of the cancer treatments had no active ingredient.
Since then, the agency has issued warnings to more than 3,000 doctors, clinics and hospitals about eight different breaches in the U.S. drug supply chain by rogue distributors such as Richards Pharma, Canada Drugs, Medical Device King, Gallant Pharmaceuticals, and TC Medical.
These rogue distributors offered 63 different non-FDA approved medicines that treated a wide variety of illnesses, including arthritis, cancer, asthma, infertility, osteoporosis, macular degeneration, Crohn’s disease, as well as IUDs, anesthesia and cosmetic injectables.
Have you or has someone you know taken medicine that these smugglers have sold? Here’s the list of drugs that appear in the FDA’s warnings:
Aclasta
Actemra
Alimta
Aloxi
Altuzan
Anzemet
Aredia
Artzal
Avastin
Boniva
Bonviva
Botox
Doxil
Dysport
Eloxatin
Erbitux
Euflexxa
Faslodex
Gemzar
Herceptin
Herclon
Hyalgan
Hydrocortistab
Implanon
Juvederm
Juvederm Ultra 2
Juvederm Ultra 3
Juvederm Ultra 4
Leucovorin
Lucentis
Macrolane
Menopur
Methotrexate
Methylprednisone
Mirena
Neulasta
Neupogen
Orencia
Orthovisc
Perlane
Perlane-L
Prolia
Propofol
Radiesse
Remicade
Restylane
Restylane-L
Ristova
Rituxan
Sandostatin-Lar
Sculptra
Supartz
Synvisc
Taxotere
Triamcinalone
Velcade
Venofer
Vidaza
Xeomin
Xolair
Zometa