The Case Against Canada Drugs
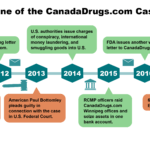
Update: On April 13, 2018, CanadaDrugs.com was sentenced to 5 years probation and to pay $34 million in fines and forfeitures for introducing misbranded drugs into interstate commerce. Two overseas subsidiaries also pleaded guilty to selling counterfeit drugs.
CEO Kris Thorkelson pleaded guilty to concealing a felony. He was sentenced to six months house arrest, five years probation and a $250,000 fine.
CanadaDrugs.com will surrender the domain names for the websites it used to illegally sell Americans drugs, and cease operations on July 13, 2018.
 In August 2015, the U.S. Department of Justice unsealed a November 2014 grand jury indictment filed against eight individuals and six companies associated with the Canadian online pharmacy giant CanadaDrugs.com. CanadaDrugs.com allegedly sold $78 million worth of unapproved, mislabeled and counterfeit cancer drugs to doctors across the U.S. between 2009 and 2012.
In August 2015, the U.S. Department of Justice unsealed a November 2014 grand jury indictment filed against eight individuals and six companies associated with the Canadian online pharmacy giant CanadaDrugs.com. CanadaDrugs.com allegedly sold $78 million worth of unapproved, mislabeled and counterfeit cancer drugs to doctors across the U.S. between 2009 and 2012.
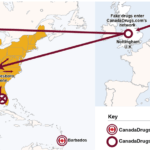
According to its own website, CanadaDrugs.com has been selling American patients imported prescription drugs–a practice that the FDA says is illegal–since 2001. The company allegedly used Barbados- and United Kingdom-based subsidiaries to expand its business to physician's offices in 2009. The company and its subsidiaries allegedly shipped illegally imported medicines which passed through the U.K. to dropshippers at three locations in the U.S.
The November 2014 indictment links CanadaDrugs.com to the distribution of two lots of counterfeit cancer medications--Avastin and Altuzan--to medical practices in the United States. It alleges that the company tried to conceal the problem rather than reporting the supply chain breach to the FDA.
The FDA's discovery of the counterfeit Avastin and Altuzan, which did not contain any active ingredient, led to a country-wide recall of Avastin in February 2012. The counterfeit cancer medications reached patients like beloved Arizona grandmother Betty Hunter, who died of lung cancer in 2011.
Since 2012, the FDA has warned more than 500 medical practices in 49 states that they have been buying misbranded drugs from subsidiaries of CanadaDrugs.com.
More News Related to the Canada Drugs Case
Learn more from PSM
The Rogues Gallery Comic Book Series: Real-life stories of fake drug criminals and their cases.

How many doctors in your state bought black market cancer drugs?