Lipo Escultura Corp. Issues Nationwide Recall of Lipo Escultura Due to Undeclared Sibutramine and Diclofenac
This is a reprint of an FDA Alert.
FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company.
December 3, 2015
Contact
Consumers
Julio Tapia (718) 415-2611 or (347) 867-9988
Firm Press Release
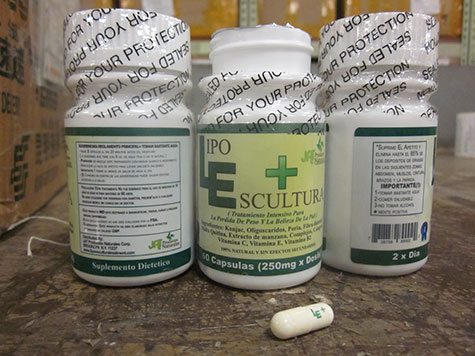
Lipo Escultura Corp. of Brooklyn, NY dba JAT Productos Naturales Corp., and JAT Natural Products Corp. are voluntarily recalling all Lipo Escultura within expiry to the consumer level. The Lipo Escultura capsules were tested by the U.S. Food and Drug Administration and have been found to contain two potentially harmful ingredients–sibutramine and diclofenac.
Risk Statement: Sibutramine is an appetite suppressant now a controlled substance that was removed from the market for safety reasons. Sibutramine is known to substantially increase blood pressure and/or pulse rate in some patients and may present a significant risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke. Diclofenac is a non-steroidal anti-inflammatory drug (commonly referred to as NSAIDs). NSAIDS may cause increased risk of cardiovascular events, such as heart attack and stroked, as well as serious gastrointestinal damage, including bleeding, ulceration, and fatal perforation of the stomach and intestines.
The product is used as a weight loss dietary supplement and is packaged in a white plastic bottle with green and lime labeling with white capsules. Products were sold/distributed Nationwide by JAT Productos Naturales Corp. via internet sales on www.lipoesculturatreatment.com, through Lipo Escultura Corp. 888 Wyckoff Ave. Brooklyn, NY 11237, a retail store and 1360 Hancock Street, Brooklyn, NY 11237, a home office.
The recall was initiated after a consumer illness was reported to the FDA and it was discovered that the product labeling does not reveal the presence of sibutramine or diclofenac.
Immediately discontinue the use of this product. Consumers with questions should contact Julio Tapia at (718) 415-2611 or (347) 867-9988 Monday through Friday from 9am to 5pm Eastern Standard Time.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting either online, by regular mail or by fax.
Online: www.fda.gov/Safety/MedWatch/default.htm
Regular Mail: use postage-paid, pre-addressed Form FDA 3500 available at: www.fda.gov/MedWatch/getforms.htm. Mail to address on the pre-addressed form.
Fax: 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.