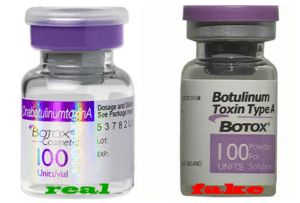
Canadian Pharmacist Sentenced For Selling Counterfeit Botox To U.S. Doctors
Source: The Counterfeit Report
The U.S. Department of Justice (DOJ) announced the sentence of a Canadian pharmacist for conspiring to distribute counterfeit, misbranded, and adulterated Botox to doctors in the U.S. Nikhil Buhecha of Vancouver, British Columbia received a sentence of 36 months. In the plea agreement, Buhecha admitted to having owned and operated a wholesale drug distribution business involving other people in Canada, Panama, and Turkey.
According to the indictment, Buhecha operated a subsidiary called “online botox,” which was a branch of a Canadian company that listed Buhecha as its director and president. Buhecha’s business purchased wholesale quantities of adulterated, misbranded, and unapproved prescription drugs from Turkey and other countries. To save on shipping costs, no temperature control devices such as dry ice or freezer packs were used when the drugs were illegally shipped into the U.S.
The drugs were shipped to post office boxes in Washington state near the Canadian border. Falsified custom forms described the contents of the boxes as “gifts” or “healthcare items and remedies for personal use,” declaring the items to have low monetary value. The drugs were picked up by other members of the conspiracy and stored in a variety of locations such as in the bedroom and refrigerator of a mobile home in Washington. The drugs were marketed to doctors in the U.S. and when orders were received, one of the conspiracy members who was storing some of the smuggled drugs received instructions on how much and to whom to ship the order to. Despite having multiple bank accounts in Canada, Buhecha set up credit card processing and banking arrangement for these transactions at a bank located in Panama. The DOJ contends this was to help hide his illegal income from both the U.S. and Canadian governments.
The FDA sent warning letters to the doctors who may have purchased the counterfeit Botox. Other prosecutions related to this case include Dr. Erick Falconer, Greg Martin, Ozkan Semizoglu, and Sabahaddin Akman. The U.S. Food and Drug Administration’s Office of Criminal Investigations investigated this case with assistance from the College of Pharmacists of British Columbia and the Royal Canadian Mounted Police, as well as INTERPOL Washington and the U.S. Marshals Service.