Fake Cancer Medicine Found To Only Contain Acetaminophen
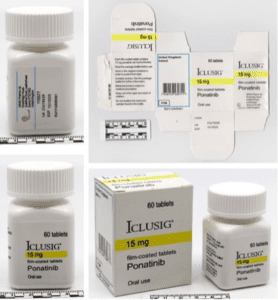
Bottle and packaging from counterfeit 15-mg Iclusig
Source: World Health Organization
The Canadian Broadcasting Corporation reported on a warning issued in North America and Europe by the World Health Organization (WHO) about two batches of counterfeit cancer drugs that testing revealed contained nothing more than over-the-counter acetaminophen. The fake drugs were packaged to look like Iclusig, an oral medication developed to treat chronic myeloid and acute lymphoblastic leukemia.
According to WHO’s warning, a Swiss wholesaler first discovered the fakes, but others have since been found in Turkey and Argentina. Testings found counterfeit pills in both 15-milligram and 45-milligram dosages. Internet sales are one of the ways that the WHO listed these pills were being distributed around the world. Takeda and Incyte are the legitimate manufacturers of Iclusig, but neither produced the counterfeits currently on the market. The batch numbers for the fakes, which do not correspond to the genuine manufacturing records, are as follows:
- Iclusig 45-milligram: Batch number PR072875 (30 tablets per bottle)
- Iclusig 15-milligram: Batch number 25A19E09 (60 tablets per bottle)
The WHO advises anyone who finds they are in possession of these counterfeit pills not to take them. U.S. citizens should report any suspicious criminal activities involving pharmaceutical medications to the U.S. Food and Drug Administration (FDA) – Office of Criminal Investigations.
Although the U.S.’s secure drug supply chain generally insulates Americans from fake and substandard medications, counterfeit medicine is estimated to be worth $200 billion per year globally. During INTERPOL’s Operation Pangea in 2018, law enforcement in 116 countries seized 500 tonnes of fake medications. For its part in the international week of action, the FDA shut down 465 websites that were selling non-FDA-approved prescription drugs including drugs for oncology, antivirals, opioids, and other untested prescription drugs to U.S. consumers.
Agencies and governments across the globe continue to struggle with how to tackle the issue of counterfeit medicine. While some believe that blockchain systems could provide the solution at some point in the future, the FDA and their European Union counterparts have both recently rolled out new track-and-trace programs to ensure the accountability of their respective drug supply chains.