Research Study Confirms That Cheap And Safe Drug Importation Is Not Feasible
In March 2020, the Journal of Pharmaceutical Health Services Research, an official journal of the Royal Pharmaceutical Society, published a study by Dr. Kristina M.L. Acri née Lybecker titled “State Pharmaceutical Importation Programmes: An Analysis of the Cost-effectiveness.” In the paper, Dr. Acri reports the results of a cost analysis of 24 prescription drugs, comparing the presumed savings of state-sponsored drug importation plans with the cost of “testing into safety” the prescription drugs procured by those plans. She also examined the cost of treating adverse events, both for the patient and for the state running the importation program.
Acri found that the cost of testing for safety and efficacy exceeded the presumed cost savings for all 24 drugs analyzed, and that depending on the imported drug, one adverse medical event could wipe out as much as a decades’ worth of projected savings.
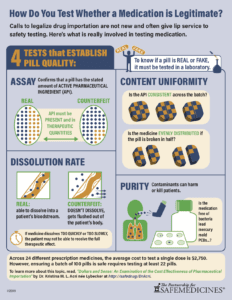
Details about testing medicine for safety and efficacy
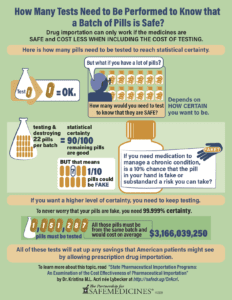
The four tests are necessary to determine whether a prescription drug is legitimate: assay, content uniformity, dissolution rate, and sterility. Acri found that the costs of testing a single sample depended on which tests a medication required, and ranged from $2,500 to $4,100. Testing a batch of pills thoroughly enough to have 90 percent confidence and 90 percent reliability that 90 out of 100 pills were legitimate, requires testing 22 pills at a cost of between $55,000 and $90,200.
To have greater than 90 percent confidence and reliability, an importation program would need to test more medicine from the same batch. To acquire the same level of confidence that American consumers currently enjoy when they pick up an FDA-approved prescription from the pharmacy, you would need to test to 99.999 percent certainty. To hit that level, you would need to test 1,151,287 samples at a cost of $2,853,217,500 to $4,679,276,700. For each drug examined, “testing into safety” dwarfed all presumed savings.
Details about Adverse Medical Events
The costs associated with adverse medical events caused by medication failure vary greatly depending on the medical reason for the treatment. For example, if someone were to receive a counterfeit version of Truvada, they would not be protected from HIV and could become infected. In 2018, the cost per patient for a single new HIV infection was $447,758. It would take 23 years and 11 months of drug importation to cover a single case of a person becoming HIV positive. Sovaldi, a drug prescribed to treat hepatitis C, had one of the longest lengths of time needed to accrue cost savings to cover an adverse medical event: more than 48 years. Of the drugs analyzed in this research study, Enbrel, a prescription drug that treats rheumatoid arthritis, had the shortest length of time to cover the cost of an adverse medical event at 65 days.
Conclusion
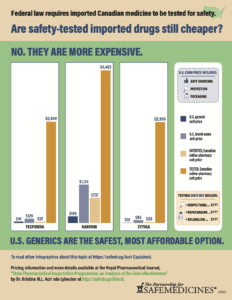
The cost to ensure the safety and quality of any imported drugs far exceeds any savings. If a single patient were to receive a counterfeit or substandard drug, the state’s drug importation program could see months, years, or decades’ worth of savings wiped out to cover the cost of just a single event. This research study demonstrated that drug importation plans can be cheap, or they can be safe, but they cannot be both.
How can importation be too expensive?
PSM's infographics explain concepts from Dr. Acri's paper, showing what kind of and how much testing is required, and how that cost outstrips any savings promised by importation.
See the latest importation news
 at the Federal and state levels: Colorado, Connecticut, Florida, Maine, New Hampshire, New Mexico, Vermont
at the Federal and state levels: Colorado, Connecticut, Florida, Maine, New Hampshire, New Mexico, Vermont
Learn more about importation:
- Importation 101 for 2021
- Report on the Potential Impact of Drug Importation Proposals on U.S. Law Enforcement (2017), and Addendum (2019)
- Statements opposing drug importation (2000 to the present)